| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| 500mg | |||
| Other Sizes |
| 靶点 |
PI3Kδ (IC50 = 24 nM); PI3Kγ (IC50 = 50 nM); PI3Kα (IC50 = 165 nM); PI3Kβ (IC50 = 215 nM)
1. Phosphatidylinositol 3-Kinase γ (PI3Kγ) and δ (PI3Kδ) - PI3Kγ (p110γ/p101 complex): IC50 ~10 nM (recombinant human PI3Kγ, HTRF-based kinase activity assay)[1] - PI3Kδ (p110δ/p85α complex): IC50 ~15 nM (recombinant human PI3Kδ, same HTRF assay)[1] 2. Low activity against other PI3K subtypes: - PI3Kα (p110α/p85α): IC50 > 1000 nM (same HTRF assay as PI3Kγ/δ)[1] - PI3Kβ (p110β/p85α): IC50 > 800 nM (same assay)[1] 3. No significant inhibition of 35+ unrelated kinases (e.g., AKT, ERK, JAK, eNOS) at 1 μM concentration[1] [1] |
|---|---|
| 体外研究 (In Vitro) |
TG100713(10 μM;48 或 72 小时)强烈抑制内皮细胞 (EC) 增殖[1]。
1. 炎症细胞功能抑制(文献[1]): - 小鼠骨髓来源中性粒细胞(BMDNs): - 100 nM TG100713 2小时抑制fMLP诱导的趋化运动~70%(Transwell实验);50 nM 15分钟减少fMLP诱导的肌动蛋白聚合~65%(鬼笔环肽染色),而肌动蛋白聚合是细胞迁移的关键步骤。 - 200 nM TG100713 24小时减少BMDNs中LPS诱导的TNF-α分泌~80%、IL-6分泌~75%(ELISA);对基础细胞因子水平无影响。 - 小鼠腹腔巨噬细胞: - 100 nM TG100713 12小时减少LPS诱导的iNOS表达~70%(Western blot);200 nM 4小时抑制巨噬细胞对FITC标记大肠杆菌的吞噬~60%(流式细胞术)。 2. PI3K信号通路抑制(文献[1]): - 血清饥饿的BMDNs与TG100713(10-500 nM)孵育1小时后,用fMLP(100 nM)刺激5分钟。50 nM TG100713 降低磷酸化AKT(Ser473)~80%、磷酸化p38 MAPK ~75%(Western blot);200 nM 完全阻断fMLP诱导的PI3K下游信号激活[1] [1] |
| 体内研究 (In Vivo) |
TG100713 显示了满足所有三个要求的最小结构,迄今为止该结构也显示出最佳的 PI3K 结合活性。有趣的是,所生成的 SAR 曲线与初始体内筛选的结果密切相关,进一步支持了 PI3K 作为抑制相关化合物血管通透性的靶标。
1. 心肌缺血再灌注(I/R)损伤保护(文献[1]): - 动物:雄性C57BL/6小鼠(8-10周龄),每组8只;适应环境7天(12小时光/暗周期,自由摄食饮水)。 - 模型建立:结扎左前降支冠状动脉(LAD)30分钟诱导心肌缺血,随后再灌注24小时。 - 给药:TG100713 溶解于10% DMSO + 90%生理盐水,腹腔注射(i.p.)10或30 mg/kg,于缺血前30分钟给药;溶媒组给予10% DMSO + 90%生理盐水。 - 药效: - 梗死面积:30 mg/kg TG100713 将心肌梗死面积从溶媒组的~45%降至~25%(p < 0.01,TTC染色);10 mg/kg 降至~35%(p < 0.05)。 - 心功能:30 mg/kg组经超声心动图检测,左心室射血分数(LVEF)改善(55±4% vs 溶媒组35±5%,p < 0.01),左心室短轴缩短率(LVFS)改善(28±3% vs 溶媒组18±2%,p < 0.01)。 - 炎症反应:30 mg/kg TG100713 较溶媒组减少梗死心肌中CD11b+中性粒细胞浸润~65%(免疫组化),血清TNF-α水平降低~70%(ELISA)[1] [1] |
| 酶活实验 |
PI3K 反应是通过使用重组人激酶、3 μM ATP、磷脂酰肌醇底物和辅因子构建的,并通过使用基于发光的检测系统来量化 ATP 消耗来测量反应进程。商业筛选服务用于进行蛋白激酶测定。
1. PI3Kγ/δ激酶活性实验(基于HTRF): - 试剂制备:重组人PI3Kγ(p110γ/p101)和PI3Kδ(p110δ/p85α)重悬于实验缓冲液(50 mM Tris-HCl pH 7.5,10 mM MgCl₂,1 mM DTT,0.01% Tween 20)。底物混合液:10 μM磷脂酰肌醇-4,5-二磷酸(PIP₂,溶于0.1% CHAPS)+ 2 μM ATP + Eu³+标记链霉亲和素-ATP。 - 反应体系:50 μL混合物含5 nM PI3Kγ/δ、底物混合液及系列浓度TG100713(0.01-1000 nM),设置溶媒对照组(0.1% DMSO)。30℃孵育60分钟,确保激酶反应充分进行。 - 检测:加入50 μL HTRF检测混合液(抗磷酸化PIP₃抗体 + XL665标记二抗),室温孵育30分钟。测定荧光(激发光337 nm,发射光620 nm(Eu³+信号)/665 nm(XL665信号))。抑制率=(1 - 药物组665/620比值 ÷ 溶媒组665/620比值)× 100%,通过非线性回归(GraphPad Prism)推导IC50。 2. 激酶选择性实验: - 试剂制备:35余种重组激酶(如AKT1、ERK2、EGFR、JAK2)重悬于各自激酶缓冲液,加入亚型特异性底物及ATP(浓度=各激酶的Km值)。 - 反应体系:25 μL混合物含10 nM激酶、底物、ATP及1 μM TG100713。30℃孵育45分钟。 - 检测:通过放射活性实验([γ-³²P]-ATP掺入)或荧光实验定量磷酸化底物,所有非PI3Kγ/δ激酶的抑制率<10%[1] [1] |
| 细胞实验 |
将人脐静脉内皮细胞(HUVEC)接种于96孔簇板(5,000个细胞/孔)中,在测定培养基(含有0.5%血清和50ng/ml VEGF)中培养,并通过XTT测定法测定其数量。存在或不存在测试化合物 (10 M).01110 位于中间位置(一些构象 ≤ 10 但一些构象 >> 50 kcal)
1. 中性粒细胞趋化实验(Transwell法): - 细胞分离:从小鼠股骨分离骨髓细胞,铺于Percoll密度梯度(40%/70%)上离心纯化中性粒细胞(BMDNs);用RPMI 1640 + 0.1% BSA重悬。 - 处理:BMDNs(1×10⁵个细胞/孔)加入Transwell上室;下室加入含fMLP(100 nM,趋化因子)的RPMI 1640 + 0.1% BSA。上、下室均加入TG100713(10-500 nM);37℃、5% CO₂孵育2小时。 - 检测:收集下室迁移细胞,台盼蓝染色后显微镜计数(每孔5个视野);迁移率=(迁移细胞数/总接种细胞数)× 100%。 2. 巨噬细胞细胞因子分泌实验(ELISA): - 细胞培养:冷PBS腹腔灌洗收集小鼠腹腔巨噬细胞,接种于24孔板(1×10⁵个/孔),用RPMI 1640 + 10% FBS过夜培养。 - 处理:与TG100713(10-500 nM)孵育1小时,再用LPS(100 ng/mL)刺激24小时。 - 检测:收集上清液,夹心ELISA法检测TNF-α和IL-6水平;根据试剂盒提供的标准曲线计算浓度。 3. PI3K信号通路Western blot实验: - 细胞培养:BMDNs接种于6孔板(2×10⁵个/孔),血清饥饿4小时。 - 处理:与TG100713(10-500 nM)孵育1小时,再用fMLP(100 nM)刺激5分钟。 - 检测:含蛋白酶/磷酸酶抑制剂的RIPA缓冲液裂解细胞;每泳道上样30 μg蛋白,10% SDS-PAGE分离。膜用抗p-AKT(Ser473)、p-p38 MAPK及内参GAPDH抗体孵育;ImageJ定量条带灰度[1] [1] |
| 动物实验 |
1. Mouse myocardial I/R injury protocol:
- Animals: Male C57BL/6 mice (8-10 weeks old) were acclimated to laboratory conditions for 7 days, with a 12-hour light/dark cycle and free access to standard chow and water. Mice were anesthetized with isoflurane (2% for induction, 1.5% for maintenance) before surgery.
- Model establishment: A left thoracotomy was performed at the 4th intercostal space; the left anterior descending coronary artery (LAD) was ligated with a 6-0 silk suture for 30 minutes (ischemia phase), confirmed by myocardial blanching. The suture was then loosened to allow 24 hours of reperfusion. Sham-operated group underwent thoracotomy without LAD ligation.
- Drug preparation: TG100713 was dissolved in a vehicle of 10% DMSO + 90% saline. The mixture was sonicated at RT for 5 minutes to ensure complete dissolution and avoid precipitation. Doses of 10 mg/kg and 30 mg/kg were prepared by adjusting the drug concentration.
- Administration: Mice were randomly divided into 3 groups (n=8/group):
- Vehicle group: I.p. injection of 10% DMSO + 90% saline (10 μL/g body weight) 30 minutes before ischemia.
- Low-dose TG100713 group: I.p. injection of 10 mg/kg TG100713 (10 μL/g body weight) 30 minutes before ischemia.
- High-dose TG100713 group: I.p. injection of 30 mg/kg TG100713 (10 μL/g body weight) 30 minutes before ischemia.
- Assessment:
- Infarct size measurement: 24 hours after reperfusion, mice were euthanized; hearts were excised, washed with cold PBS, and cut into 1-mm transverse slices. Slices were stained with 2% TTC (37℃, 15 minutes) to distinguish infarcted (pale) and viable (red) myocardium. Infarct size was calculated as (infarcted area / total left ventricular area) × 100%.
- Cardiac function measurement: Transthoracic echocardiography was performed 24 hours after reperfusion using a high-frequency ultrasound system. LVEF and LVFS were calculated from M-mode images.
- Inflammation assessment: Serum was collected for TNF-α ELISA; infarcted myocardial tissue was fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with anti-CD11b antibody for neutrophil counting[1]
[1] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. In vitro toxicity:
- BMDNs, peritoneal macrophages, and normal human umbilical vein endothelial cells (HUVECs): TG100713 at concentrations up to 1 μM showed no non-specific cytotoxicity. LDH release assay revealed <10% leakage (vs. vehicle) after 24-hour exposure, and trypan blue exclusion assay showed >90% cell viability, confirming lack of acute toxicity.
2. In vivo toxicity:
- Mice (i.p. administration of 10-30 mg/kg TG100713 for 24 hours): No mortality or abnormal behaviors (e.g., ataxia, lethargy, reduced food/water intake) were observed. Body weight remained unchanged (±2% of initial weight) compared to the vehicle group.
- Serum chemistry: 24 hours after drug administration, serum levels of ALT/AST (liver function) and creatinine (kidney function) were within normal ranges (ALT: 50 ± 6 U/L vs. normal 40-60 U/L; AST: 118 ± 10 U/L vs. normal 100-130 U/L; creatinine: 56 ± 4 μmol/L vs. normal 50-70 μmol/L, n=5 per group).
|
| 参考文献 | |
| 其他信息 |
1. Mechanism of action:
TG100713 is a selective PI3Kγ/δ dual inhibitor that binds to the ATP-binding pockets of p110γ and p110δ catalytic subunits. This binding blocks PI3Kγ/δ-mediated phosphorylation of PIP₂ to PIP₃, thereby inhibiting downstream signaling pathways (AKT, p38 MAPK) critical for inflammatory cell (neutrophil, macrophage) activation, migration, and cytokine secretion. In myocardial I/R injury, reduced inflammation cell infiltration and cytokine release alleviate myocardial necrosis, ultimately reducing infarct size and preserving cardiac function[1]
2. Preclinical significance: - Identifies TG100713 as a potential therapeutic agent for myocardial I/R injury, a major cause of mortality in acute myocardial infarction. Its ability to target PI3Kγ/δ (key regulators of inflammatory responses) addresses the unmet need for anti-inflammatory therapies that protect myocardium without compromising normal immune function. - Demonstrates that pre-ischemia administration of TG100713 effectively reduces infarct size, supporting its potential use in clinical settings (e.g., before percutaneous coronary intervention [PCI] for acute myocardial infarction)[1] 3. Limitations: - No clinical development data (e.g., FDA approval status) reported; TG100713 remains a preclinical research tool compound. - Efficacy was only evaluated in mouse myocardial I/R models; no data in large animal models (e.g., pigs, rabbits) or human clinical samples[1] [1] |
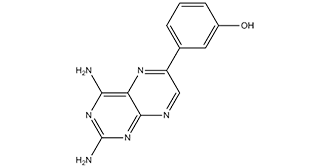
| 分子式 |
C12H10N6O
|
|
|---|---|---|
| 分子量 |
254.2474
|
|
| 精确质量 |
254.091
|
|
| 元素分析 |
C, 56.69; H, 3.96; N, 33.05; O, 6.29
|
|
| CAS号 |
925705-73-3
|
|
| 相关CAS号 |
|
|
| PubChem CID |
17751063
|
|
| 外观&性状 |
Light yellow to khaki solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
646.4±65.0 °C at 760 mmHg
|
|
| 闪点 |
344.8±34.3 °C
|
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
|
| 折射率 |
1.815
|
|
| LogP |
0.5
|
|
| tPSA |
124.56
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
19
|
|
| 分子复杂度/Complexity |
317
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
OC1C=C(C2C=NC3N=C(N)N=C(C=3N=2)N)C=CC=1
|
|
| InChi Key |
UOORQSPLBHUQDQ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C12H10N6O/c13-10-9-11(18-12(14)17-10)15-5-8(16-9)6-2-1-3-7(19)4-6/h1-5,19H,(H4,13,14,15,17,18)
|
|
| 化学名 |
3-(2,4-diaminopteridin-6-yl)phenol
|
|
| 别名 |
TG100713; TG-100713; TG 100713
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~2 mg/mL (~7.9 mM)
Water: <1 mg/mL Ethanol: <1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9331 mL | 19.6657 mL | 39.3314 mL | |
| 5 mM | 0.7866 mL | 3.9331 mL | 7.8663 mL | |
| 10 mM | 0.3933 mL | 1.9666 mL | 3.9331 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|