| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
托瑞米芬是第二代选择性雌激素受体调节剂 (SERM),正在开发用于阻止骨质疏松症和 ADT 诱导的前列腺癌的其他副作用 [1]。他莫昔芬、托瑞米芬和阿他美坦体外抑制Ac-1细胞的增殖,IC50值分别为1.8±1.3μM、1±0.3μM和60.4±17.2μM。根据体外研究,托瑞米芬和阿他美坦的组合比单独使用任何一种药物更有效[3]。
|
|---|---|
| 体内研究 (In Vivo) |
之后,利用在卵巢切除的雌性 SCID 小鼠中开发的 Ac-1 异种移植物在体内研究了这种组合的影响。将托瑞米芬(1000μg/天)、阿他美坦(1000μg/天)、他莫昔芬(100μg/天)或托瑞米芬和阿他美坦的组合注射到小鼠体内。这项调查的结果表明,托瑞米芬加阿他美坦与单独使用任一药物一样有效,尽管可能并不比单独使用任一药物更有效。使用他莫昔芬没有其他好处[3]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Well absorbed Toremifene is extensively metabolized, principally by CYP3A4 to N-demethyltoremifene, which is also antiestrogenic but with weak in vivo antitumor potency. 580 L 5 L/h Metabolism / Metabolites Hepatic. Mainly by CYP3A4 to N-demethyltoremifene, which exhibits antiestrogenic effects but has weak antitumor potency in vivo. Toremifene has known human metabolites that include N-desmethyltoremifene. Biological Half-Life 5 days |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Toremifene has been associated with mild-to-moderate serum ALT or AST elevations in 5% to 19% of patients, but these abnormalities are usually transient and not associated with symptoms or jaundice. Elevations above 5 times the ULN are uncommon ( Likelihood score: D (possible cause of clinically apparent liver injury). Protein Binding Toremifen is primarily bound to albumin (92%), 2% bound to α1-acid glycoprotein, and 6% bound to β1-globulin in the serum. |
| 参考文献 |
|
| 其他信息 |
Toremifene is a tertiary amine, an organochlorine compound and an aromatic ether. It has a role as an antineoplastic agent, an estrogen antagonist, an estrogen receptor modulator and a bone density conservation agent. It derives from a hydride of a stilbene.
Toremifene is a selective estrogen receptor modulator (SERM) and a nonsteroidal antiestrogen used to treat estrogen receptor positive breast cancer. Like [tamoxifen], toremifene is part of the first-generation triphenylethylene derivative chemical class of SERMs. Toremifene possesses tissue-specific actions: it has estrogenic (agonist) activity on the cardiovascular system and on bone tissue and it has weak estrogenic effects on uterine tissue, however, it also has antiestrogenic (estrogen-antagonist) activity on breast tissue. Toremifene is an Estrogen Agonist/Antagonist. The mechanism of action of toremifene is as a Selective Estrogen Receptor Modulator. Toremifene is a nonsteroidal antiestrogen that is used in the treatment of estrogen receptor positive breast cancer. Long term toremifene therapy has been associated with development of fatty liver, steatohepatitis, cirrhosis, and rare instances of clinically apparent acute liver injury. Toremifene is a nonsteroidal triphenylethylene antiestrogen. Chemically related to tamoxifen, toremifene is a selective estrogen receptor modulator (SERM). This agent binds competitively to estrogen receptors, thereby interfering with estrogen activity. Toremifene also has intrinsic estrogenic properties, which are manifested according to tissue type or species. (NCI04) A first generation selective estrogen receptor modulator (SERM). Like TAMOXIFEN, it is an estrogen agonist for bone tissue and cholesterol metabolism but is antagonistic on mammary and uterine tissue. See also: Toremifene Citrate (has salt form). Drug Indication For the treatment of metastatic breast cancer in postmenopausal women with estrogen receptor-positive or receptor-unknown tumors. Toremifene is currently under investigation as a preventative agent for prostate cancer in men with high-grade prostatic intraepithelial neoplasia and no evidence of prostate cancer. First line hormone treatment of hormone-dependent metastatic breast cancer in postmenopausal patients. Fareston is not recommended for patients with estrogen receptor negative tumours. Mechanism of Action Toremifene is a nonsteroidal triphenylethylene derivative. Toremifene binds to estrogen receptors and may exert estrogenic, antiestrogenic, or both activities, depending upon the duration of treatment, animal species, gender, target organ, or endpoint selected. The antitumor effect of toremifene in breast cancer is believed to be mainly due to its antiestrogenic effects, in other words, its ability to compete with estrogen for binding sites in the cancer, blocking the growth-stimulating effects of estrogen in the tumor. Toremifene may also inhibit tumor growth through other mechanisms, such as induction of apoptosis, regulation of oncogene expression, and growth factors. Pharmacodynamics Toremifene is an antineoplastic hormonal agent primarily used in the treatment of advanced breast cancer. Toremifene is a nonsteroidal agent that has demonstrated potent antiestrogenic properties in animal test systems. The antiestrogenic effects may be related to its ability to compete with estrogen for binding sites in target tissues such as breast. Toremifene inhibits the induction of rat mammary carcinoma induced by dimethylbenzanthracene (DMBA) and causes the regression of already established DMBA-induced tumors. In this rat model, Toremifene appears to exert its antitumor effects by binding the estrogen receptors. In cytosols derived from human breast adenocarcinomas, Toremifene competes with estradiol for estrogen receptor protein. |
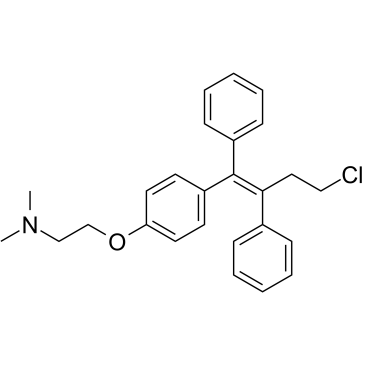
| 分子式 |
C26H28CLNO
|
|---|---|
| 分子量 |
405.97
|
| 精确质量 |
405.185
|
| CAS号 |
89778-26-7
|
| 相关CAS号 |
Toremifene citrate;89778-27-8;Toremifene-d6;Toremifene-d6 hydrochloride
|
| PubChem CID |
3005573
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
535.1±50.0 °C at 760 mmHg
|
| 熔点 |
108-110°C
|
| 闪点 |
277.4±30.1 °C
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
| 折射率 |
1.588
|
| LogP |
7.82
|
| tPSA |
12.47
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
483
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CN(C)CCOC1=CC=C(C=C1)/C(=C(/CCCl)\C2=CC=CC=C2)/C3=CC=CC=C3
|
| InChi Key |
XFCLJVABOIYOMF-QPLCGJKRSA-N
|
| InChi Code |
InChI=1S/C26H28ClNO/c1-28(2)19-20-29-24-15-13-23(14-16-24)26(22-11-7-4-8-12-22)25(17-18-27)21-9-5-3-6-10-21/h3-16H,17-20H2,1-2H3/b26-25-
|
| 化学名 |
Ethanamine, 2-(4-((1Z)-4-chloro-1,2-diphenyl-1-butenyl)phenoxy)-N,N-dimethyl-
|
| 别名 |
GTx 006 Z-Toremifene Acapodene Farestone
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4632 mL | 12.3162 mL | 24.6324 mL | |
| 5 mM | 0.4926 mL | 2.4632 mL | 4.9265 mL | |
| 10 mM | 0.2463 mL | 1.2316 mL | 2.4632 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。