| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| Other Sizes |
|
| 靶点 |
GnRH/Gonadotropin-releasing hormone
|
|---|---|
| 体外研究 (In Vitro) |
曲普瑞林对雷公藤多苷诱导的小鼠卵巢细胞损伤具有保护作用 [2]。
|
| 体内研究 (In Vivo) |
曲普瑞林对雷公藤引起的雌性小鼠卵巢功能损害具有保护作用[2]。
|
| 动物实验 |
For qualified, healthy SD female mice, the vaginal exfoliated cell method was used to select 30 mice with normal estrous cycle as test animals, which were randomly divided into 3 groups of 10 mice each: • Group A: blank control group, where 0.35 mL of saline was administered to the stomach once daily for 11 weeks; • Group B: tripterygium glycoside group, where 0.35 mL of tripterygium glycoside solution was administered to the stomach from the 8th day; once a day for 10 weeks; • Group C: triptolide + triptorelin group: 0.1 mg/kg daily subcutaneous injection of triptorelin injection; once a day; continuous injection for 11 weeks; from the 8th day, the triptolide solution was administered to the stomach 0.35 mL, once daily for 10 weeks. From the first day of the experiment, the general conditions of the mice were observed and recorded, including energy, activity, hair, food intake, water intake, stomach appetite, second stool, etc. The mice were weighed once a week to observe changes in body weight. The vagina exfoliation cell method, simple to operate, was used to observe the estrous cycle. After 11 weeks of treatment, the drug was stopped for 3 weeks and all mice were sacrificed. The ovaries were then obtained by laparotomy, and the ovarian wet weight was measured using an electronic analytical balance. The ovarian index was calculated by ovarian wet weight (mg) / mouse weight (g) × 100%. After weighing, the ovaries were fixed in a 4% paraformaldehyde solution for 3 days, and were routinely dehydrated, xylene-transparented, wax-impregnated, embedded, sectioned (4 µm), and operated according to the instructions of immunohistochemistry kit. Immunohistochemical average optical density (average optical) analysis method: each slice in each group randomly selected at least three positions with a 200× field of view (FoV) for photographing. When taking pictures, FoV was selected to ensure that the testing tissue fully filled the view. In addition, the background illumination of each photo was kept as consistent as possible. Image-Pro Plus 6.0 software was used to select the same brown-yellow color as the uniform standard for determining the positives of all photos. Each photo was analyzed to obtain the Integrated Optical Density (IOD) and the pixel area (AREA). The average optical density (AO) was obtained by AO = IOD/AREA. (1) The larger the AO value, the higher the positive expression level. [2]
|
| 参考文献 |
[1]. Efficacy and safety of triptorelin 6-month formulation in patients with central precocious puberty.2016 Nov 1;29(11):1241-1248.
[2]. www.ejgo.net/articles/10.31083/j.ejgo.2021.02.2299 |
| 其他信息 |
Triptorelin Pamoate is the pamoate salt of triptorelin, a synthetic decapeptide agonist analog of luteinizing hormone releasing hormone (LHRH). Possessing greater potency than endogenous LHRH, triptorelin reversibly represses gonadotropin secretion after prolonged administration. After chronic, continuous administration, a sustained decrease in LH, FSH and testicular and ovarian steroidogenesis is observed. The serum testosterone concentration may fall to levels typically seen in surgically castrated men. (NCI04)
A potent synthetic long-acting agonist of GONADOTROPIN-RELEASING HORMONE with D-tryptophan substitution at residue 6. See also: Triptorelin Pamoate (annotation moved to). |
| 分子式 |
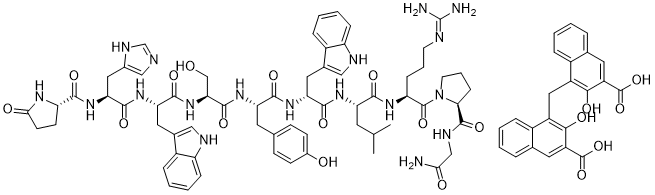
C87H98N18O19
|
|---|---|
| 分子量 |
1699.85
|
| 精确质量 |
1698.725
|
| 元素分析 |
C, 61.47; H, 5.81; N, 14.83; O, 17.88
|
| CAS号 |
124508-66-3
|
| 相关CAS号 |
140194-24-7 (acetate);57773-63-4;124508-66-3 (pamoate);2240176-35-4 (TFA);
|
| PubChem CID |
25074469
|
| 外观&性状 |
Solid powder
|
| tPSA |
606
|
| 氢键供体(HBD)数目 |
21
|
| 氢键受体(HBA)数目 |
21
|
| 可旋转键数目(RBC) |
37
|
| 重原子数目 |
124
|
| 分子复杂度/Complexity |
3280
|
| 定义原子立体中心数目 |
9
|
| SMILES |
N.NC(NCCCC(C(CN1CCCC1C(NCC(=O)N)=O)=O)NC(C(NC(C(CC1=CCC2=CC=CC=C12)NC(C(NC(C(NC(C(NC(C(CC(C1CCC(=O)N1)=O)CC1=CN=CN1)=O)CCC1=CC=CC=C1NC=C)=O)CO)=O)CC1=CC=C(O)C=C1)=O)=O)CC(C)C)=O)=N.OC(C1=CC2=CC=CC=C2C(CC2=C(O)C(C(=O)O)=CC3=CC=CC=C23)=C1O)=O
|
| InChi Key |
ZBVJFYPGLGEMIN-OYLNGHKZSA-N
|
| InChi Code |
InChI=1S/C64H82N18O13.C23H16O6/c1-34(2)23-46(56(88)75-45(13-7-21-69-64(66)67)63(95)82-22-8-14-52(82)62(94)72-31-53(65)85)76-58(90)48(25-36-28-70-42-11-5-3-9-40(36)42)78-57(89)47(24-35-15-17-39(84)18-16-35)77-61(93)51(32-83)81-59(91)49(26-37-29-71-43-12-6-4-10-41(37)43)79-60(92)50(27-38-30-68-33-73-38)80-55(87)44-19-20-54(86)74-4424-20-16(14-7-3-1-5-12(14)9-18(20)22(26)27)11-17-15-8-4-2-6-13(15)10-19(21(17)25)23(28)29/h3-6,9-12,15-18,28-30,33-34,44-52,70-71,83-84H,7-8,13-14,19-27,31-32H2,1-2H3,(H2,65,85)(H,68,73)(H,72,94)(H,74,86)(H,75,88)(H,76,90)(H,77,93)(H,78,89)(H,79,92)(H,80,87)(H,81,91)(H4,66,67,69)1-10,24-25H,11H2,(H,26,27)(H,28,29)/t44-,45-,46-,47-,48+,49-,50-,51-,52-/m0./s1
|
| 化学名 |
(S)-1-((3S,6S,9S,12S,15R,18S,21S)-3-((1H-imidazol-5-yl)methyl)-6,15-bis((1H-indol-3-yl)methyl)-21-(3-((diaminomethylene)amino)propyl)-12-(4-hydroxybenzyl)-9-(hydroxymethyl)-18-isobutyl-1,4,7,10,13,16,19-heptaoxo-1-((S)-5-oxopyrrolidin-2-yl)-2,5,8,11,14,17,20-heptaazadocosan-22-oyl)-N-(2-amino-2-oxoethyl)pyrrolidine-2-carboxamide
4,4'-methylenebis(3-hydroxy-2-naphthoate)
|
| 别名 |
AY25650 Wy42462 CL118532AY-25650 Wy-42462CL-118532 AY 25650CL 118532 Wy4 2462 TriptorelinD-Trp-6-LH-RH Trelstar
Depot. Decapeptyl Decapeptyl Depot Decapeptyl LP Decapeptyl
Trimestral Embonate.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: > 10mM
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.5883 mL | 2.9414 mL | 5.8829 mL | |
| 5 mM | 0.1177 mL | 0.5883 mL | 1.1766 mL | |
| 10 mM | 0.0588 mL | 0.2941 mL | 0.5883 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Study to Assess the Efficacy and Safety of the Triptorelin 6-month Formulation in Paediatric Participants With Central Precocious Puberty.
CTID: NCT05029622
Phase: Phase 3 Status: Completed
Date: 2024-06-13