| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |

| 体外研究 (In Vitro) |
钠离子维生素 C 转运蛋白 2 (SVCT-2) 是 L-抗坏血酸的转运蛋白,决定了其抗癌作用。根据 L-抗坏血酸饮食和 SVCT-2 表达,L-抗坏血酸 (0.1 μM–2 mM) 具有抗癌作用。人类结直肠癌细胞对 L-抗坏血酸的敏感性各不相同,主要取决于 SVCT-2 表达的程度 [4]。 L-抗坏血酸 (10 μg) 和 L-抗坏血酸 (50 μg/ml,5 d) 可促进 IPSC 重编程 [5]。 L-抗坏血酸(50 μg/ml,9 天)促进成纤维细胞转化为心肌细胞[6]。 (50 ng/ml,4-6 天)促进小鼠的终末分泌 B 细胞发育为全能 IPS 细胞[7]。
|
|---|---|
| 体内研究 (In Vivo) |
L-抗坏血酸和甲苯磺丁脲的组合旨在在正常(60 毫克/公斤)和糖尿病(40 毫克/公斤)环境下产生降血压作用。与甲苯磺丁脲对照相比,在 L-抗坏血酸存在下,托布米特 (20 mg/kg) 的作用持续时间更长,作用开始时间更早 [5]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
70% to 90% The efficiency of absorption depends on the salt form, the amount administered, the dosing regimen and the size of iron stores. Subjects with normal iron stores absorb 10% to 35% of an iron dose. Those who are iron deficient may absorb up to 95% of an iron dose. Ascorbic acid is readily absorbed from the gastrointestinal tract and is widely distributed in the body tissues. Plasma concentrations of ascorbic acid rise as the dose ingested is increased until a plateau is reached with doses of about 90 to 150 mg daily. Body stores of ascorbic acid in health are about 1.5 g although more may be stored at intakes above 200 mg daily. The concentration is higher in leucocytes and platelets than in erythrocytes and plasma. In deficiency states the concentration in leucocytes declines later and at a slower rate, and has been considered to be a better criterion for the evaluation of deficiency than the concentration in plasma. Ascorbic acid is reversibly oxidized to dehydroascorbic acid; some is metabolized to ascorbate-2-sulfate, which is inactive, and oxalic acid which are excreted in the urine. Ascorbic acid in excess of the body's needs is also rapidly eliminated unchanged in the urine; this generally occurs with intakes exceeding 100 mg daily. Ascorbic acid crosses the placenta and is distributed into breast milk. It is removed by hemodialysis. The renal threshold for ascorbic acid is approx 14 ug/mL, but this level varies among individuals. When the body is saturated with ascorbic acid and blood concentrations exceed the threshold, unchanged ascorbic acid is excreted in the urine. When tissue saturation and blood concentrations of ascorbic acid are low, administration of the vitamin results in little or no urinary excretion of ascorbic acid. Inactive metabolites of ascorbic acid such as ascorbic acid-2-sulfate and oxalic acid are excreted in the urine ... Ascorbic acid is also excreted in the bile but there is no evidence for enterohepatic circulation ... For more Absorption, Distribution and Excretion (Complete) data for L-Ascorbic Acid (29 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic. Ascorbic acid is reversibly oxidised (by removal of the hydrogen from the enediol group of ascorbic acid) to dehydroascorbic acid. The two forms found in body fluids are physiologically active. Some ascorbic acid is metabolized to inactive compounds including ascorbic acid-2-sulfate and oxalic acid. Ascorbic acid-2-sulfate has ... been identified as metabolite of Vitamin C in human urine. Ascorbate is oxidized to CO2 in rats and guinea pigs, but considerably less conversion can be detected in man. One route of metabolism of the vitamin in man involves its conversion to oxalate and eventual excretion in the urine; dehydroascorbate is presumably an intermediate. ... Young male guinea pigs /were fed/ diets containing either 2 g/kg (18 control animals) or 86 g/kg (29 treatment animals) of ascorbic acid for 275 days. The average weight gain was significantly higher in the control group. Eight control and eight treatment animals, chosen to maintain comparable weights between the groups, were then given a totally deficient ascorbic acid diet 24 hr before a metabolic study was initiated. In the metabolic study, (14)C-labeled L-ascorbic acid (628 g) was then injected intraperitoneally into both treatment and control guinea pigs to study the catabolism and excretion of the ascorbic acid. Catabolism of the labeled ascorbic acid to respiratory (14)CO2 was increased in treatment guinea pigs. The control and treatment animals were then divided into two groups. One group received 3 mg/kg ascorbic acid (chronic deficiency) for 68 days. The other received a diet devoid of ascorbic acid (acute deficiency) for 44 days. Four control and three treatment animals from the chronic deficiency group and three control and four treatment animals from the acute deficiency group were given a totally deficient ascorbic acid diet 24 hr before a second metabolic study was initiated. (14)C-labeled L-ascorbic acid (628 g) was injected intraperitoneally as above. Treatment animals in the chronic deficiency and the acute deficiency groups had increased catabolism of the labeled ascorbic acid to respiratory (14)CO2 compared to control animals in the chronic and acute deficiency groups. The amount of radioactivity recovered in the urine and feces was similar for both groups except for an increased urinary excretion of the label in treated animals exposed to the totally deficient diet. The treatment animals maintained higher tissue stores of ascorbic acid than the control animals. However, this difference was significant only in the testes. When subjected to a totally deficient diet the treatment animals were depleted of ascorbic acid at a faster rate than the control animals. The accelerated catabolism was not reversible by subnormal intakes of the vitamin ... ... Hartley guinea pigs approximately 30 days pregnant /were divided/ into a control group receiving 25 mg ascorbic acid and a treated group receiving 300 mg/kg/day ascorbic acid daily. All animals were fed a 0.05% ascorbic acid diet. The groups were maintained for 10 days on their respective diets. Pups (both sexes) were randomly chosen on either day 5 or day 10 for the metabolic study. L-l-(14)C-Ascorbic Acid (10 uCi/mM) was injected intraperitoneally into the pups and they were placed in a metabolic chamber for five hours to collect expired (14)CO2. From day 11 all pups were caged individually and weaned to a diet containing only traces of ascorbic acid. Every third day the animals were examined for physical signs of scurvy. Once signs appeared, the animals were examined daily until death. Necropsies were performed on all animals. Pups from the treated group demonstrated a marked increase in (14)CO2 excretion following the intraperitoneal injection. Signs of scurvy appeared 4 days earlier in the treated group and mortality of the treated pups occurred approximately one week earlier. When excretion of labeled CO2 in both groups was correlated with the day of onset of scurvy signs, a linear correlation was found between the two parameters, suggesting that the earlier appearance of signs of scurvy on the experimental pups is secondary to an increased rate of ascorbic acid catabolism ... For more Metabolism/Metabolites (Complete) data for L-Ascorbic Acid (10 total), please visit the HSDB record page. Ascorbic acid has known human metabolites that include Ascorbic acid-2-sulfate. Biological Half-Life 16 days (3.4 hours in people who have excess levels of vitamin C) The plasma half-life is reported to be 16 days in humans. This is different in people who have excess levels of vitamin C where the half-life is 3.4 hours Vitamin C has a 96 hr half-life in guinea pigs. Due to homeostatic regulation, the biological half-life of ascorbate varies widely from 8 to 40 days and is inversely related to the ascorbate body pool. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Origin of the substance: Ascorbic acid is of both natural and synthetic origin. Natural origin: ascorbic acid is found in fresh fruit and vegetables. Citrus fruits are a particularly good source of ascorbic acid and also hip berries, acerola and fresh tea leaves. Ascorbic acid exists as colorless, or white or almost white crystals. It is odorless or almost odorless. It has a pleasant, sharp acidic taste. It is freely soluble in water and sparingly soluble in ethanol. It is practically insoluble in ether and chloroform. HUMAN EXPOSURE: Main risks and target organs: The main target organs for toxicity are found in the gastrointestinal, renal and hematological systems. Summary of clinical effects: In individuals with glucose-6-phosphate dehydrogenase (G-6-PD) deficiency, hemolytic anemia may develop after administration of ascorbic acid. In individuals predisposed to renal stones, chronic administration of high doses may lead to renal calculi formation. In some cases, acute renal failure may be observed under both conditions. Indications: Prevention and treatment of scurvy. It has been used as a urinary acidifier and in correcting tyrosinemia in premature infants on high-protein diets. The drug may be useful to treat idiopathic methemoglobinemia. Contraindications: Ascorbic acid is contraindicated in patients with hyperoxaluria and G-6-PD deficiency. Routes of entry: Oral: Ascorbic acid is usually administered orally in extended-release capsule form, tablets, lozenges, chewable tablets, solutions and extended-release tablets and capsules Absorption by route of exposure: Ascorbic acid is readily absorbed after oral administration but the proportion does decrease with the dose. GI absorption of ascorbic acid may be reduced in patients with diarrhea or GI diseases. Distribution by route of exposure: Normal plasma concentrations of ascorbic acid are about 10 to 20 ug/mL. Total body stores of ascorbic acid have been estimated to be about 1.5 g with about a 30 to 45 mg daily turnover. Plasma concentrations of ascorbic acid rise as the dose ingested is increased until a plateau is reached with doses of about 90 to 150 mg daily. Ascorbic acid becomes widely distributed in body tissues with large concentrations found in the liver, leukocytes, platelets, glandular tissues, and the lens of the eye. In the plasma about 25% of the ascorbic acid is bound to proteins. Ascorbic acid crosses the placenta; cord blood concentration are generally 2 to 4 times the concentration in maternal blood. Ascorbic acid is distributed into milk. In nursing mothers on a normal diet the milk contains 40 to 70 ug/mL of the vitamin. Biological half-life by route of exposure: The plasma half-life is reported to be 16 days in humans. This is different in people who have excess levels of vitamin C where the half-life is 3.4 hours. Metabolism: Ascorbic acid is reversibly oxidized to dehydroascorbic acid in the body. This reaction, which proceeds by removal of the hydrogen from the enediol group of ascorbic acid, is part of the hydrogen transfer system. The two forms found in body fluids are physiologically active. Some ascorbic acid is metabolized to inactive compounds including ascorbic acid-2-sulfate and oxalic acid. Elimination by route of exposure: The renal threshold for ascorbic acid is approximately 14 ug/mL, but this level varies among individuals. When the body is saturated with ascorbic acid and blood concentrations exceed the threshold, unchanged ascorbic acid is excreted in the urine. When tissue saturation and blood concentrations of ascorbic acid are low, administration of the vitamin results in little or no urinary excretion of ascorbic acid. Inactive metabolites of ascorbic acid such as ascorbic acid-2-sulfate and oxalic acid are excreted in the urine. Ascorbic acid is also excreted in the bile but there is no evidence for enterohepatic circulation. Pharmacology and toxicology: Mode of action: Toxicodynamics: Hyperoxaluria may result after administration of ascorbic acid. Ascorbic acid may cause acidification of the urine, occasionally leading to precipitation of urate, cystine, or oxalate stones, or other drugs in the urinary tract. Urinary calcium may increase, and urinary sodium may decrease. Ascorbic acid reportedly may affect glycogenolysis and may be diabetogenic but this is controversial. Pharmacodynamics: In humans, an exogenous source of ascorbic acid is required for collagen formation and tissue repair. Vitamin C is a co-factor in many biological processes including the conversion of dopamine to noradrenaline, in the hydroxylation steps in the synthesis of adrenal steroid hormones, in tyrosine metabolism, in the conversion of folic acid to folinic acid, in carbohydrate metabolism, in the synthesis of lipids and proteins, in iron metabolism, in resistance to infection, and in cellular respiration. Vitamin C may act as a free oxygen radical scavenger. Toxicity: Human data: Adults: Diarrhea may occur after oral dosage of large amounts of ascorbic acid. Interactions: Concurrent administration of more than 200 mg of ascorbic acid per 300 mg of elemental iron increases absorption of iron from the GI tract. Increased urinary excretion of ascorbic acid and decreased excretion of aspirin occur when the drugs are administered concurrently. Ascorbic acid increases the apparent half-life of paracetamol. Interference with anticoagulant therapy has been reported. Carcinogenicity: It has been reported that there is no evidence of carcinogenicity. Some studies suggest that vitamin C may amplify the carcinogenic effect of other agents. L-ascorbic acid increases the oral carcinoma size induced by dimethylbenz(a)anthracene. Also, butylated hydroxyanisole induced forestomach carcinogenesis in rats. Teratogenicity: There is no evidence of teratogenicity. Mutagenicity: Ascorbic acid is reported to increase the rate of mutagenesis in cultured cells but this only occurs in cultures with elevated levels of Cu(2+) or Fe(2+). This effect may be due to the ascorbate induced generation of oxygen-derived free radicals. However, there is no evidence of ascorbate induced mutagenesis in vivo. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Vitamin C is a normal component of human milk and is a key milk antioxidant. The recommended vitamin C intake in lactating women is 120 mg daily, and for infants aged 6 months or less is 40 mg daily. High daily doses up to 1000 mg increase milk levels, but not enough to cause a health concern for the breastfed infant and is not a reason to discontinue breastfeeding. Nursing mothers may need to supplement their diet to achieve the recommended intake or to correct a known deficiency. Maternal doses of vitamin C in prenatal vitamins at or near the recommended intake do not alter milk levels. Freezing (-20 degrees C) freshly expressed mature milk from hospitalized mothers of term and preterm infants does not change milk vitamin C levels for at least 3 months of freezer storage. After 6 to 12 months of freezing (-20 degrees C), vitamin C levels can decrease by 15 to 30%. Storage at -80 degrees C preserves vitamin C levels for up to 8 months, with 15% loss by 12 months. ◉ Effects in Breastfed Infants Sixty healthy lactating women between 1 and 6 months postpartum exclusively breastfeeding their infants were given vitamin C 500 mg plus vitamin E 100 IU once daily for 30 days, or no supplementation. Infants of supplemented mothers had increased biochemical markers of antioxidant activity in their urine. Clinical outcomes were not reported. Eighteen preterm infants, seven of whom were less than 32 weeks gestational age, who were fed pooled, Holder-pasteurized donor milk beginning during the first three days of life had their average blood plasma ascorbic acid concentrations decrease from 15.5 mg/L at birth to 5.4 mg/L by 1 week of age, and to 4.1 mg/L by 3 weeks of age. The authors described the 1- and 3-week levels as subtherapeutic (<6 mg/L) and indicative of inadequate intake, potentially jeopardizing postnatal growth potential. Although this study was conducted before advances in the provision of parenteral nutrition and enteral milk fortification for preterm infants, contemporary studies suggest that inadequate vitamin C intake from pooled, pasteurized donor milk may be a potential health problem for preterm infants receiving donor milk. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 25% Interactions BALB/c male mice (288) were allocated into four groups: group 1 (48 animals), control diet; group 2 (48 animals), control diet and 500 ppm 2-acetylaminofluorene (2-AAF); group 3 (96 animals), control diet and 250 mg/mL of ascorbic acid in water; group 4 (96 animals), control diet, 2-AAF, and ascorbic acid. Food and water consumptions were measured at weekly intervals. The animals were killed at 28 days and necropsied. There were no detectable differences in relative food consumption due to the addition of ascorbic acid or to an interaction of ascorbic acid with 2-AAF. However the presence of ascorbic acid in the water was associated with a significant reduction in relative water consumption. The addition of 2-AAF caused a significant increase in relative water consumption, and a significant interaction of ascorbic acid with 2-AAF was detected. Major histological findings were restricted to the urinary bladder. Vacuolization of the transitional epithelium, simple and nodular urothelial hyperplasia, fibrosis, and chronic inflammation of the lamina propria were found in varying degrees in the urinary bladders of mice receiving 2-AAF alone and in combination with ascorbic acid. The most severe lesions were seen in the mice given the combination of 2-AAF and ascorbic acid. The urinary bladders of mice receiving the control diet and ascorbic acid alone were normal. The chronic inflammation and fibrosis were restricted primarily to the fundus of the urinary bladder. The lamina propria contained an increased amount of collagen, an increase in the vasculature and an infiltration of mononuclear inflammatory cells ... Effect of ascorbic acid on metal toxicity. Table: Effect of Ascorbic Acid on Metal Toxicity [Table#2228] Non-Human Toxicity Values LD50 Rat oral 11,900 mg/kg LD50 Rat oral > 5000 mg/kg bw /From table/ LD50 Rat sc 5,000 mg/kg bw /From table/ LD50 Rat iv 1,000 mg/kg bw /From table/ For more Non-Human Toxicity Values (Complete) data for L-Ascorbic Acid (24 total), please visit the HSDB record page. |
| 参考文献 |
[1]. Michael T Nelson, et al. Molecular mechanisms of subtype-specific inhibition of neuronal T-type calcium channels by ascorbate. J Neurosci. 2007 Nov 14;27(46):12577-83.
[2]. Aleksander Hinek, et al. Sodium L-ascorbate enhances elastic fibers deposition by fibroblasts from normal and pathologic human skin. J Dermatol Sci. 2014 Sep;75(3):173-82. [3]. Sungrae Cho, et al. Hormetic dose response to L-ascorbic acid as an anti-cancer drug in colorectal cancer cell lines according to SVCT-2 expression. Sci Rep. 2018 Jul 27;8(1):11372. [4]. Satyanarayana Sreemantula, et al. Influence of antioxidant (L- ascorbic acid) on tolbutamide induced hypoglycaemia/antihyperglycaemia in normal and diabetic rats. BMC Endocr Disord. 2005 Mar 3;5(1):2. [5]. Sebastian J Padayatty, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003 Feb;22(1):18-35. [6]. Esteban MA, Wang T, Qin B, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6(1):71-79. doi:10.1016/j.stem.2009.12.001 [7]. Talkhabi M, Pahlavan S, Aghdami N, Baharvand H. Ascorbic acid promotes the direct conversion of mouse fibroblasts into beating cardiomyocytes. Biochem Biophys Res Commun. 2015;463(4):699-705. [8]. Stadtfeld M, Apostolou E, Ferrari F, et al. Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nat Genet. 2012;44(4):398-S2. |
| 其他信息 |
Therapeutic Uses
Antioxidants; Free Radical Scavengers Prophylaxis and treatment of scurvy Ascorbic acid 100 to 200 mg daily may be given with desferrioxamine in the treatment of patients with thalassemia, to improve the chelating action of desferrioxamine, thereby increasing the excretion of iron. In iron deficiency states ascorbic acid may increase gastrointestinal iron absorption and ascorbic acid or ascorbate salts are therefore included in some oral iron preparations. For more Therapeutic Uses (Complete) data for L-Ascorbic Acid (30 total), please visit the HSDB record page. Drug Warnings Large doses are reported to cause diarrhea and other gastrointestinal disturbances. It has also been stated that large doses may result in hyperoxaluria and the formation of renal calcium oxalate calculi, and ascorbic acid should therefore be given with care to patients with hyperoxaluria. Tolerance may be induced with prolonged use of large doses, resulting in symptoms of deficiency when intake is reduced to normal. Prolonged or excessive use of chewable vitamin C preparations may cause erosion of tooth enamel. Large doses of ascorbic acid have resulted in hemolysis in patients with G6PD deficiency. Vitamin C intakes of 250 mg/day or higher have been associated with false-negative results for detecting stool and gastric occult blood. Therefore, high dose vitamin C supplements should be discontinued at least two weeks before physical exams to avoid interference with blood and urine tests. Supplemental vitamin C may reduce the effectiveness of cancer chemotherapy, and its effectiveness in reducing risk from cancer and related death is unclear. For more Drug Warnings (Complete) data for L-Ascorbic Acid (25 total), please visit the HSDB record page. Pharmacodynamics Ascorbic Acid (vitamin C) is a water-soluble vitamin indicated for the prevention and treatment of scurvy, as ascorbic acid deficiency results in scurvy. Collagenous structures are primarily affected, and lesions develop in bones and blood vessels. Administration of ascorbic acid completely reverses the symptoms of ascorbic acid deficiency. The major activity of supplemental iron is in the prevention and treatment of iron deficiency anemia. Iron has putative immune-enhancing, anticarcinogenic and cognition-enhancing activities. |
| 分子式 |
C6H8O6
|
|---|---|
| 分子量 |
176.12
|
| 精确质量 |
176.032
|
| CAS号 |
50-81-7
|
| 相关CAS号 |
L-Ascorbic acid;50-81-7;L-Ascorbic acid sodium salt;134-03-2;L-Ascorbic acid calcium dihydrate;5743-28-2;L-Ascorbic acid;50-81-7
|
| PubChem CID |
54670067
|
| 外观&性状 |
Crystals (usually plates, sometimes needles, monoclinic system)
White crystals (plates or needles) White to slightly yellow crystals or powder ... gradually darkens on exposure to light |
| 密度 |
2.0±0.1 g/cm3
|
| 沸点 |
552.7±50.0 °C at 760 mmHg
|
| 熔点 |
190-194 °C (dec.)
|
| 闪点 |
238.2±23.6 °C
|
| 蒸汽压 |
0.0±3.4 mmHg at 25°C
|
| 折射率 |
1.711
|
| LogP |
-2.41
|
| tPSA |
107.22
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
12
|
| 分子复杂度/Complexity |
232
|
| 定义原子立体中心数目 |
2
|
| SMILES |
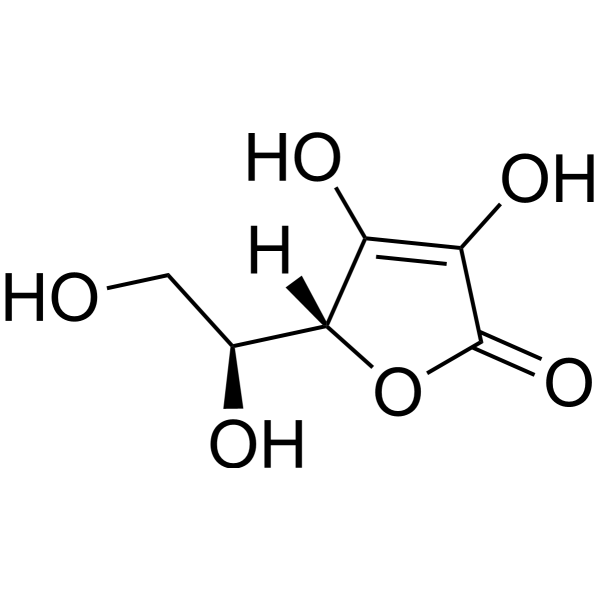
O1C(C(=C([C@@]1([H])[C@]([H])(C([H])([H])O[H])O[H])O[H])O[H])=O
|
| InChi Key |
CIWBSHSKHKDKBQ-JLAZNSOCSA-N
|
| InChi Code |
InChI=1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2,5,7-10H,1H2/t2-,5+/m0/s1
|
| 化学名 |
(2R)-2-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxy-2H-furan-5-one
|
| 别名 |
ascorbate; Vitamine C; L-ascorbic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~567.79 mM)
H2O : ≥ 100 mg/mL (~567.79 mM) |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.6779 mL | 28.3897 mL | 56.7795 mL | |
| 5 mM | 1.1356 mL | 5.6779 mL | 11.3559 mL | |
| 10 mM | 0.5678 mL | 2.8390 mL | 5.6779 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Vitamin C as add-on Therapy in Patients With Acute Herpes Zoster
CTID: NCT05561257
Phase: Phase 2 Status: Terminated
Date: 2024-11-07