| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
KRAS G12C
|
|---|---|
| 体外研究 (In Vitro) |
结束了一个被药物开发失败所破坏的时代,人们对直接靶向KRAS的兴趣减弱,并认为难以解决,新技术和策略正在帮助目标的复苏。如前所述,四氢吡啶并嘧啶被鉴定为KRASG12C的不可逆共价抑制剂,它们结合在KRAS的开关II口袋中,与半胱氨酸12形成共价键。使用基于结构的药物设计,结合集中的体外吸收、分布、代谢和排泄筛选方法,合成了类似物,以提高该系列的效力并降低代谢责任。描述了临床开发候选物MRTX849作为KRASG12C的强效选择性共价抑制剂的发现[1]。
|
| 酶活实验 |
KRASG12C目标参与度[1]
肿瘤片段在6 M胍-HCl、50 mM N-(2-羟乙基)哌嗪-N′-乙磺酸(HEPES)(pH 7.5)和5 mM TCEP中均化。离心后,使用Bradford测定法测定上清液的蛋白质浓度。将内标物(13C15N重组KRASG12C)和20 mM碘乙酰胺加入200μL裂解缓冲液中的200μg肿瘤蛋白中,并在37°C下在黑暗中孵育样品30分钟。烷基化后,使用96孔Zeba旋转板将100μL反应物交换为1 M胍-HCl、50 mM HEPES(pH 7.5)。在37°C下用1μg胰蛋白酶/Lys-C混合物消化蛋白质18小时。使用C18旋转板对肽进行脱盐,并通过蒸发去除溶剂。将肽溶解在0.1%甲酸、5%乙腈、95%水中,用于LCMS分析。使用Sciex TripleTOF仪器上的靶向方法监测含Cys-12的KRASG12C肽(一种内部参考肽)以及相应的同位素标记肽。KRASG12C的参与度是按照之前的报告计算的。 |
| 细胞实验 |
基于细胞的磷酸化ERK检测[1]
所有实验均在标准条件下(37°C和5%CO2)进行。采用四参数法通过剂量反应曲线拟合计算IC50值。将携带KRASG12C突变的NCI-H358细胞接种在补充有10%胎牛血清的RPMI中的96孔板中。将培养皿孵育过夜。用抑制剂孵育细胞3小时后,用磷酸缓冲盐水(PBS)洗涤细胞一次,用3.8%甲醛固定,用冰冷的甲醇渗透。然后将平板与Li-Cor阻断缓冲液一起孵育。随后,通过与针对GAPDH(小鼠)和磷酸化ERK(兔)的一抗孵育,通过细胞内蛋白质法评估ERK的磷酸化。然后将平板与小鼠或兔特异性的荧光二抗一起孵育。在Li-Cor荧光平板阅读器上以680和800nm波长对平板进行成像。将磷酸化ERK信号归一化为GAPDH信号,并生成POC值[1]。 |
| 动物实验 |
In Vivo Studies[1]
Mice were maintained under pathogen-free conditions, and food and water were provided ad libitum. 6–8 week-old, female, athymic nude-Foxn1nu mice were injected subcutaneously with either NCI-H358 or MIA PaCa-2 cells in 100 μL of PBS and Matrigel matrix in the right hind flank with 5.0 × 106 cells 50:50 cells/Matrigel. Mouse health was monitored daily, and caliper measurements began when tumors were palpable. Tumor volume measurements were determined utilizing the formula 0.5 × L × W2 in which L refers to length and W refers to the width of each tumor. When tumors reached an average tumor volume of ∼350 mm3, mice were randomized into treatment groups. Mice were treated by oral gavage with either vehicle consisting of 10% research grade Captisol in 50 mM citrate buffer pH 5.0 or study compound in vehicle at indicated doses. For efficacy studies, animals were orally administered study compound or vehicle and monitored daily, tumors were measured 3 times per week and body weights were measured 2 times per week. Study day on efficacy plots indicates the day after which compound treatment was initiated. For PK/PD studies, tumors and plasma were collected after a single dose at the time points and concentration range indicated. |
| 参考文献 |
[1]. Jay B Fell, et al. Identification of the Clinical Development Candidate MRTX849, a Covalent KRAS G12C Inhibitor for the Treatment of Cancer. J Med Chem. 2020 Jul 9;63(13):6679-6693.
|
| 其他信息 |
Stability—Liver Cytosol Supplemented with GSH[1]
Compounds (1 μM) were incubated at 37 °C for 30 min in a 1 mg/mL liver cytosol, supplemented with 5 mM GSH in KPB buffer. At the end of the designated time point, samples were quenched with acetonitrile spiked with 40 mM NEM and 0.2 μM concentration of labetalol (internal standard). Samples were centrifuged, and supernatants were analyzed by LC–MS/MS. Whole Blood Stability[1] Compounds (5 μM) were incubated at 37 °C for 30, 60, and 240 min in a 1:1 (v/v) blood and PBS, pH 7.4. Diluted blood was preincubated for 15 min at 37 °C and 100% humidity before the reactions were initiated with the dosing of the compound. At the end of each designated time point, the red blood cells were lysed 1:1 with water, mixed at 600 rpm for 1 min, and stopped with acetonitrile containing 0.625 μM labetalol (internal standard). Samples were centrifuged, and supernatants were analyzed by LC–MS/MS. Measurement of kinact/KI[1] Recombinant KRASG12C “Lite” (C51S/C80L/C118S) was reacted with a range of MRTX849 concentrations in 25 mM HEPES (pH 7.0), 150 mM NaCl, 5 mM MgCl2, 10 mM octyl β-glucopyranoside, and 0.5 mM TCEP, for 0–45 s, at room temperature. At each time-point, the reaction was quenched with 50 mM HCl, and 0.25 μg of pepsin was added. KRASG12C was digested for 4 h at 37 °C, and the resulting Cys-12-containing peptide was analyzed by LCMS. The percent of modified KRASG12C at each time-point was calculated from the 0 s control sample for each concentration of MRTX849, and kobs was subsequently calculated from the slope of the ln(POC) versus time data. Rate versus concentration data fit the Michaelis–Menten equation. Metabolite Identification in Hepatocytes[1] Cryopreserved hepatocytes from mouse, rat, dog, and human were thawed and diluted to a viable cell density of 1 × 106 cells/mL using Dulbecco’s modified Eagle medium. MRTX849 (10 μM) was added to 1 mL of each hepatocyte suspension, and samples were incubated at 37 °C for 2 h. Metabolism was quenched with the addition of 1% formic acid, samples were centrifuged, and the supernatant was loaded onto a 3cc Oasis HLB cartridge. MRTX849 and metabolites were washed with 5% methanol in water and eluted with 100% methanol. The solvent was evaporated under a stream of nitrogen gas, and metabolites were reconstituted with 150 μL of 30:70 acetonitrile/water (v/v). Metabolites were separated on a Gemini NX-C18 column and detected by absorbance at 290 nm and mass spectrometry. Relative amounts of metabolites were determined by A290nm peak intensity, and the biotransformation was characterized by the associated m/z and MSMS fragmentation patterns. NCI-H358 Proteome Cysteine Selectivity Assay[1] NCI-H358 cells were treated with 3 μM MRTX849 for 3 h and then lysed with 1% NP-40-containing buffer and sonication. Cell extracts were treated with 5 mM iodoacetamide desthiobiotin for 1 h at room temperature. Proteins were precipitated with acetone and resuspended in a buffer containing 6 M guanidine–HCl, 50 mM HEPES, pH 7.5, and 5 mM TCEP and incubated for 15 min at 65 °C. Following treatment with iodoacetamide and buffer exchange into 1 M guanidine-HCl, 50 mM HEPES (pH 7.5), proteins were digested with trypsin/Lys-C overnight. Desthiobiotinylated peptides were enriched using streptavidin agarose and eluted with 50% acetonitrile and 0.1% TFA. The solvent was removed by evaporation and peptides were resuspended in 0.1% formic acid, 5% acetonitrile, and in water. Samples were analyzed on a Sciex 6600 TripleTOF instrument. Protein Pilot 5 was used to identify peptides labeled with desthiobiotin from data-dependent MS/MS scans and relative quantitation (MTRX849-treated vs control) was conducted using SWATH analysis. Click Chemistry Target Identification[1] Two flasks of NCI-H358 cells (60 million cells/T225 flask) in the RPMI 1640 SILAC light media were treated with DMSO as the control, and two flasks of cells in the RPMI 1640 SILAC heavy media (13C6-lysine, 13C6,15N4-arginine) were treated with 1 μM MRTX849 for 3 h at 37 °C. All cells were then treated with 2 μM compound 24 for 3 h at 37 °C. Cells were then lysed with 50 mM HEPES, 150 NaCl, 0.5% Triton-X 100, 1 mM EDTA, 1 mM EGTA, and HALT protease inhibitor cocktail, for 5 min, probe sonicated, and centrifuged, and the supernatant was filtered with a 45 μm syringe filter. For each replicate, 4 mg of protein in “light”’ lysate was combined with 4 mg of protein in “heavy” lysate for sample processing. Proteins were isolated using chloroform/methanol precipitation, resuspended in 0.18% sodium dodecyl sulfate (SDS) in 50 mM HEPES pH 7.5, and ‘clicked’ to azide agarose by incubating with 5 mM ascorbate, 1 mM CuSO4, and 2 mM BTTAA, for 2.5 h at room temperature. Resin-bound proteins were treated with 10 mM DTT for 40 min and then with 20 mM iodoacetamide for 30 min and washed with (1) 100 mM Tris, pH 8.0, 250 mM NaCl, 1% SDS, 5 mM EDTA, (2) 8 M urea, 100 mM Tris, pH 8.0, and (3) 20% acetonitrile in water. Trypsin/Lys-C (1 μg) was added to the protein-bound resin, suspended in 20 mM Tris pH 8.0, 2 mM CaCl2, 10% acetonitrile, and incubated for 18 h at 37 °C. Eluted peptides were desalted on C18 spin columns, solvent was removed by evaporation, and peptides were resuspended in 0.1% formic acid, 5% acetonitrile, and in water for LCMS analysis. Samples were analyzed on a Sciex 6600 TripleTOF instrument. Protein Pilot 5 was used to identify peptides from data-dependent MS/MS scans of an unlabeled sample, and relative quantitation (MTRX849-treated vs control) was conducted using SWATH analysis of the SILAC samples. |
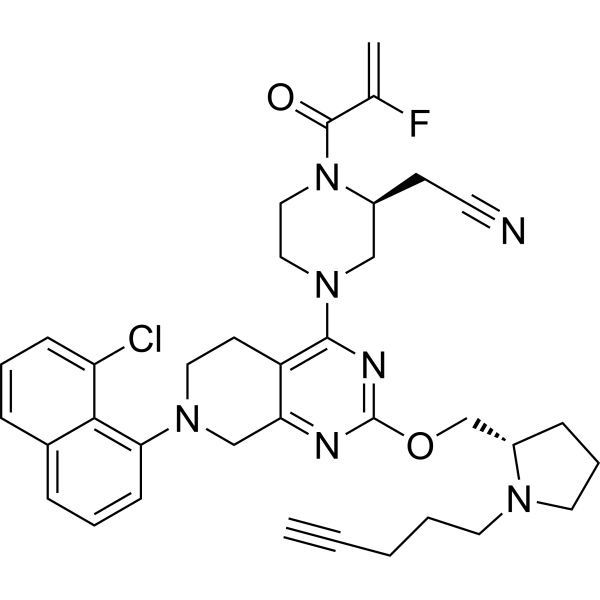
| 分子式 |
C36H39CLFN7O2
|
|---|---|
| 分子量 |
656.19
|
| 精确质量 |
655.283
|
| CAS号 |
2490716-96-4
|
| 相关CAS号 |
Adagrasib;2326521-71-3
|
| PubChem CID |
162642619
|
| 外观&性状 |
Typically exists as White to off-white solid at room temperature
|
| LogP |
6
|
| tPSA |
88.8Ų
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
47
|
| 分子复杂度/Complexity |
1200
|
| 定义原子立体中心数目 |
2
|
| SMILES |
C=C(C(=O)N1CCN(C[C@@H]1CC#N)C2=NC(=NC3=C2CCN(C3)C4=CC=CC5=C4C(=CC=C5)Cl)OC[C@@H]6CCCN6CCCC#C)F
|
| InChi Key |
YUPCFANWZUHYAJ-NSOVKSMOSA-N
|
| InChi Code |
InChI=1S/C36H39ClFN7O2/c1-3-4-5-17-42-18-8-11-28(42)24-47-36-40-31-23-43(32-13-7-10-26-9-6-12-30(37)33(26)32)19-15-29(31)34(41-36)44-20-21-45(35(46)25(2)38)27(22-44)14-16-39/h1,6-7,9-10,12-13,27-28H,2,4-5,8,11,14-15,17-24H2/t27-,28-/m0/s1
|
| 化学名 |
2-[(2S)-4-[7-(8-chloronaphthalen-1-yl)-2-[[(2S)-1-pent-4-ynylpyrrolidin-2-yl]methoxy]-6,8-dihydro-5H-pyrido[3,4-d]pyrimidin-4-yl]-1-(2-fluoroprop-2-enoyl)piperazin-2-yl]acetonitrile
|
| 别名 |
MRTX-849 analog 24; MRTX849 analog 24
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~76.20 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5239 mL | 7.6197 mL | 15.2395 mL | |
| 5 mM | 0.3048 mL | 1.5239 mL | 3.0479 mL | |
| 10 mM | 0.1524 mL | 0.7620 mL | 1.5239 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。