| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
IC50: 0.25 nM (LSD1)[1]
|
|---|---|
| 体外研究 (In Vitro) |
CC-90011(化合物 11)在 AML kasumi-1 细胞中表现出强大的抗增殖作用,EC50 为 2 nM,并且在 THP-1 细胞系中强烈激活靶向细胞分化标记物 CD11b [1]。当 CC-90011 给药四天时,在药理学相关浓度下以剂量依赖性方式观察到 GRP 抑制(EC50=3 nM,H209 和 4 nM,H1417)。当 SCLC 细胞用 CC-90011 处理 12 天时,它们表现出很强的抗增殖活性 (EC50=6 nM,H1417),这与 GRP 的抑制有关[1]。
|
| 体内研究 (In Vivo) |
在患者来源的异种移植 SCLC 模型中,CC-90011(5 mg/kg;口服;每天;持续 30 天)治疗可抑制肿瘤生长[1]。在 SCLC 人类肿瘤异种移植 (H1417) 小鼠中,CC-90011(每天一次;持续 4 天)治疗导致 GRP mRNA 水平在 2.5 mg/kg 时强烈下调,并在 5 mg/kg 时最大抑制 GRP[1]。 CC-90011(化合物 11;5 mg/kg)具有 7.5 L/kg 的高分布容积,消除半衰期为 2 小时,静脉给药后的全身清除率为 32.4 mL/min/kg。口服 CC-90011(化合物 11;5 mg/kg)可产生高度的口服生物利用度(口服生物利用度 = 32%,AUC0-24h = 1.8 μM·h,C/sub>max = 0.36 μM)。
|
| 酶活实验 |
化合物11(CC-90011)对LSD1的酶抑制作用通过LSD1单独或LSD1-CoREST复合物进行评估。[1]
对于LSD1 TR-FRET测定,LSD1(最终0.025 nM)与50 nM H3K4me1在50 mM HEPES(pH 7.3)、10 mM NaCl、0.5 mM TCEP、0.02%(w/v)BSA、0.005%(w/v)Brij-35和2µM FAD中混合,在DMSO或DMSO中的化合物稀释系列(最终1%DMSO)存在下进行。在LANCE检测缓冲液中,在LSD1抑制剂如1.8 mM盐酸环丙环丙胺(2-PCPA)的存在下,通过添加检测试剂Phycolink Streptavidin别藻蓝蛋白和铕抗未修饰组蛋白H3赖氨酸4(H3K4)抗体,将去甲基化产物H3K4定量至最终浓度分别为12.5 nM和0.25 nM 对于LSD1-CoREST测定,使用HRP偶联测定法检测酶反应产物过氧化氢。LSD1 CoREST(最终6 nM)与20µM H3K4me2、2 U/ml HRP、50µM Amplex red在50 mM HEPES(pH 7.3)、10 mM NaCl、0.02%(w/v)BSA、0.005%(w/v)Brij-35和2µM FAD混合,在DMSO或DMSO中的化合物稀释系列(最终1%DMSO)存在下进行。检测到HRP和过氧化氢反应产生的荧光产物Resorufin。 LSD2亚型和单胺氧化酶(MAO)选择性:[1] 评估了CC-90011对其他含FAD酶的抑制作用:LSD2、MAO-A和MAO-B。化合物11对LSD1的选择性大于这些酶的60000倍(表S1) 通过TR-FRET测定评估CC-90011对LSD2的酶抑制作用。在DMSO或DMSO中的化合物稀释系列(最终1%DMSO)的存在下,将LSD2(2nM最终)与300nM H3K4me1在50mM Tris pH 8.5、0.02%(w/v)BSA、0.005%(w/v)Brij-35和2µM FAD中混合。脱甲基产物H3K4以与LSD1 TR-FRET测定相同的方式进行定量,Phycolink Streptavidinalophycocyanin的终浓度为25 nM,铕抗未修饰组蛋白H3赖氨酸4(H3K4)抗体的终浓度是0.5 nM。 类似于LSD1-CoREST测定的HRP偶联测定用于MAO-A和MAO-B。在MAO-A测定中,将30µM酪胺和5µg/ml MAO-A与化合物或DMSO对照(最终1%)混合,并在2 U/ml HRP、50 mM HEPES(pH 7.3)中的50µM Amplex red、10 mM NaCl、0.02%(w/v)BSA和0.005%(w/v)Brij-35存在下进行监测 在MAO-B测定中,将300µM苄胺和5µg/ml MAO-B与化合物或DMSO对照(最终1%)混合,并在2 U/ml HRP、50 mM HEPES(pH 7.3)中的50µM Amplex red、10 mM NaCl、0.02%(w/v)BSA和0.005%(w/v)Brij-35存在下进行监测。 |
| 细胞实验 |
体外细胞生物学:化合物11(CC-90011)在AML的Kasumi-1细胞系模型中显示出强大的抗增殖活性,IC50为0.0024μM(图S1A),在正常人成纤维细胞模型中没有显示出任何作用(IC50>10μM)(图S1B)。[1]
使用基于Cell MTS比色板的测定来定量化合物11存在或不存在时新产生的NADH的量。NADH水平被用作细胞增殖定量的指标。在37°C、5%CO2的条件下,将细胞在CC-90011的11点稀释系列中孵育168小时。在该化合物培养结束时,加入CellTiter 96®非放射性细胞增殖测定水溶液(Promega),并测定OD490。IC50值使用IDBS Xlfit软件包计算,包括背景减除OD490值和DMSO对照标准化。Cell Titer Glo发光细胞活力测定(Promega)基于ATP的定量测量活细胞的数量。细胞在化合物11的8点稀释系列存在下孵育,AML细胞系在37°C、5%CO2下孵育7天,H1417细胞系孵育12天。在孵育结束时,将Cell Titer Glo试剂加入孔中,读取平板的发光情况。使用IDBS Xlfit软件包计算IC50值。[1] CC-90011:CD11b对THP1中CD11b表达的调节被证明在LSD1抑制下上调。为了评估化合物11抑制AML中LSD1的能力,利用定量FACS测定AML细胞系THP-1中CD11b蛋白的表达。在该试验中,化合物11增加了CD11b蛋白表达,EC50值为7 nM(图S2)。[1] THP-1 CD11b:细胞与化合物11一起孵育96小时。通过FACS分析细胞CD11b表达。CD11b阳性细胞相对于化合物11浓度的百分比以Xlfit绘制,以产生EC50值。 |
| 动物实验 |
Animal/Disease Models: BALB/c nude mice bearing small cell lung carcinoma (SCLC)[1]
Doses: 5 mg/kg Route of Administration: Oral administration; daily; for 30 days Experimental Results: demonstrated a tumor growth inhibition (TGI) of 78% at 5 mg /kg with no body weight loss. In vivo PK/PD analysis of GRP expression in SCLC: [1] Nude mice implanted with the SCLC cell line H1417 tumor were dosed orally daily with 2.5, 5, and 10 mg/kg compound 11 (CC-90011) for 4 consecutive days (3 mice for each group). Tumors, which were approximately 100 mm3 at the initiation of the study, were harvested 24h following the last dose. Total RNA was used to make cDNA for qPCR assessment. Total levels of human GRP transcript were normalized to human RPL19 levels. Quantification of target inhibition was generated by calculating Ct values of compound 11 treated vs vehicle GRP transcript. In vivo efficacy of compound 11 (CC-90011) in H1417 SCLC xenograft: [1] The efficacy and tolerability of compound 11, dosed orally at 2.5 and 5 mg/kg, was evaluated in the NCIH1417 small cell lung cancer (SCLC) xenograft model in athymic nude mice. Mean tumor growth in the control progressed over the course of the study exhibited a 2.5 fold increase in mean tumor volume from Day 0 to Day 65. Tumors in the compound 11 (CC-90011) treated groups regressed after Day 14, resulting in a net loss in mean tumor volume at study end. The differences in the distribution of tumor volumes on Day 65 for compound 11 treated versus control animals were significant with a calculated probability (p) ≤ 0.001 and ≤ 0.0001 for the 2.5 and 5 mg/kg dose levels, respectively. Compound 11 appeared well tolerated, and animals receiving the 2.5 or 5 mg/kg doses exhibited respective mean body weight gains of 1% and 7.5% at study end (Figure S4). All animals survived the duration of the study. In vivo efficacy in LXFS 615 SCLC PDX model: [1] Compound 11 (CC-90011) was shown to be efficacious in LXFS 615 patient derived xenograft model of SCLC with a TGI of 78% at 5 mg/kg (P-value: 0.001). Compound 11 was well tolerated with no body weight loss (Figure S5). [1] In vivo efficacy of SCLC PDX model (LU2514): Compound 11 (CC-90011) when dosed orally for 28 days was shown to be efficacious in LU-2514 patient derived xenograft model of SCLC with a TGI of 56% at 10 mg/kg (P-value: 0.0024) and 39% at 5 mg/kg (P-value: 0.0109). Compound 11 was well tolerated at all doses examined in this study with mean body weight losses <10%. |
| 参考文献 | |
| 其他信息 |
Pulrodemstat is an orally available inhibitor of lysine specific demethylase 1 (LSD1), with potential antineoplastic activity. Upon administration, pulrodemstat binds to and inhibits LSD1, a demethylase that suppresses the expression of target genes by converting the di- and mono-methylated forms of lysine at position 4 of histone H3 (H3K4) to mono- and unmethylated H3K4, respectively. LSD1 inhibition enhances H3K4 methylation and increases the expression of tumor (remove hyphen) suppressor genes. This may lead to an inhibition of cell growth in LSD1-overexpressing tumor cells. In addition, LSD1 demethylates mono- or di-methylated H3K9 which increases gene expression of tumor promoting genes; inhibition of LSD1 promotes H3K9 methylation and decreases transcription of these genes. LSD1, an enzyme belonging to the flavin adenine dinucleotide (FAD)-dependent amine oxidase family that is overexpressed in certain tumor cells, plays a key role in tumor cell growth and survival.
Histone demethylase LSDl (KDMlA) belongs to the flavin adenine dinucleotide (FAD) dependent family of monoamine oxidases and is vital in regulation of mammalian biology. Dysregulation and overexpression of LSD1 are hallmarks of a number of human diseases, particularly cancers that are characterized as morphologically poorly differentiated. As such, inhibitors of LSD1 have potential to be beneficial as a cancer therapy. The most clinically advanced inhibitors of LSDl are covalent inhibitors derived from tranylcypromine (TCP). Herein, we report the discovery of a novel series of reversible and selective LSDl inhibitors. Exploration of structure-activity relationships (SARs) and optimization of ADME properties resulted in the identification of clinical candidate CC-90011. CC-90011 exhibits potent on-target induction of cellular differentiation in acute myeloid leukemia (AML) and small cell lung cancer (SCLC) cell lines, and antitumor efficacy in patient-derived xenograft (PDX) SCLC models. CC-90011 is currently in phase 2 trials in patients with first line, extensive stage SCLC (ClinicalTrials.gov identifier: NCT03850067).[1] Conventional targeted agents for combating cancers have focused on promoting apoptosis in rapidly proliferating cancer cells. These agents have proven effective in reducing tumor bulk, but prolonged treatment has often resulted in tumors developing resistance. An alternative strategy is to suppress proliferation by inducing terminal differentiation of the cancer cells. This strategy is employed less frequently but has been highly effective in subsets of AML. The discovery of CC-90011, a highly potent and reversible inhibitor of LSD1, provides a novel differentiation strategy for the treatment of neuroendocrine tumors and AML. Phase 1 study of CC-90011 in patients with advanced solid tumors has been completed, and the safety, tolerability, and preliminary efficacy have been reported. CC-90011 is currently in phase 2 trials in patients with first line, extensive stage SCLC (ClinicalTrials.gov identifier NCT03850067).[1] |
| 分子式 |
C31H31F2N5O5S
|
|---|---|
| 分子量 |
623.67
|
| 精确质量 |
623.201
|
| 元素分析 |
C, 59.70; H, 5.01; F, 6.09; N, 11.23; O, 12.83; S, 5.14
|
| CAS号 |
2097523-57-2
|
| 相关CAS号 |
Pulrodemstat benzenesulfonate;2097523-60-7;Pulrodemstat;1821307-10-1
|
| PubChem CID |
139600313
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
158
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
44
|
| 分子复杂度/Complexity |
1070
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
OZZFOHIBJFKYLY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C24H23F2N5O2.C7H8O3S/c1-30-23(32)21(14-5-6-20(33-2)19(26)11-14)22(15-3-4-16(13-27)18(25)12-15)29-24(30)31-9-7-17(28)8-10-31;1-6-2-4-7(5-3-6)11(8,9)10/h3-6,11-12,17H,7-10,28H2,1-2H3;2-5H,1H3,(H,8,9,10)
|
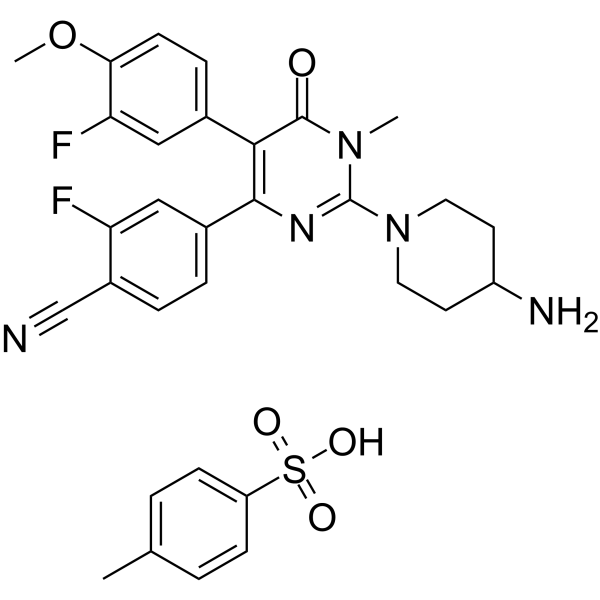
| 化学名 |
4-[2-(4-aminopiperidin-1-yl)-5-(3-fluoro-4-methoxyphenyl)-1-methyl-6-oxopyrimidin-4-yl]-2-fluorobenzonitrile;4-methylbenzenesulfonic acid
|
| 别名 |
097523-57-2; Pulrodemstat tosylate; UNII-496P6HY485; Pulrodemstat (Methylbenzenesulfonate); 496P6HY485; Benzonitrile, 4-(2-(4-amino-1-piperidinyl)-5-(3-fluoro-4-methoxyphenyl)-1,6-dihydro-1-methyl-6-oxo-4-pyrimidinyl)-2-fluoro-, 4-methylbenzenesulfonate (1:1); Pulrodemstat Methylbenzenesulfonate;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6034 mL | 8.0171 mL | 16.0341 mL | |
| 5 mM | 0.3207 mL | 1.6034 mL | 3.2068 mL | |
| 10 mM | 0.1603 mL | 0.8017 mL | 1.6034 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。