| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| Other Sizes |
| 靶点 |
CA IX/carbonic anhydrase (IC50 = 30 nM)
|
|---|---|
| 体外研究 (In Vitro) |
乙酰唑酰胺同样抑制 hCA II,IC50 为 130 nM [1]。乙酰唑酰胺 (Ace) 是一种杂芳基淀粉酰胺,其体积很小,对多种生物环酶具有高亲和力。它可用作生物环淀粉酶(CA)的染料。当低浓度乙酰唑酰胺钠 (AceL, 10 nM) 与高浓度乙酰唑酰胺钠 (AceH, 50 nM)、顺铂 (Cis; 1 μg/mL) 和 Cis 混合时,Hep Vitality - 2 细胞显着减少加载[2]。与明天相比,乙酰唑胺/Cis 联合治疗显着增加了 P53 表达水平,因为 AceL+Cis 和 AceH+Cis 治疗也导致 P53 表达水平显着增加。此外,Ace/Cis联合处理显着降低了bcl-2/bax的表达并增加了caspase-3蛋白的表达。 Ace和Cis一起可以有效增加Hep-2细胞。与监测相比,AceL、AceH、Cis 和 AceL+Cis 治疗显着降低了 bcl-2 [2]。当Ace和Cis一起使用时,可以大大降低Hep-2细胞中AQP1 mRNA的表达。与实验室相比,AceH 和 AceL+Cis 处理均降低了 Hep-2 细胞中水通道蛋白 1 (AQP1) mRNA 的表达 [2]。
Acetazolamide (AZ) /乙酰唑胺, MS-275和AZ + MS-275处理抑制NB SH-SY5Y细胞的生长。 AZ、MS-275和AZ + MS-275处理降低NB SH-SY5Y细胞的迁移能力。 结果:我们评估了HDAC抑制剂(HDACi) pyridylmethyl-N-{4-[(2-氨基苯基)-氨基甲酰]-benzyl}-氨基甲酸酯(MS-275)与pan CA抑制剂乙酰唑酰胺(acetazolamide, AZ)联合对NB SH-SY5Y、SK-N-SH和SK-N-BE(2)细胞的抗肿瘤潜力。关键观察结果是AZ + MS-275联合用药显著抑制NB细胞株SH-SY5Y的生长,诱导细胞周期阻滞和凋亡,降低迁移能力。[2] 本研究的目的是确定Acetazolamide (AZ) /乙酰唑胺(Ace)治疗是否增强Hep-2喉部细胞对顺铂(Cis)的化疗敏感性。在对数生长期,Hep-2细胞分别用Ace、Cis或两者处理,用MTT法检测细胞活力。流式细胞术检测细胞凋亡程度。western blotting检测凋亡相关蛋白BCL2凋亡调节因子(bcl-2)、BCL2相关X (bax)和caspase-3的表达水平,增殖相关蛋白增殖细胞核抗原(PCNA)和肿瘤蛋白p53 (p53)的表达水平。采用逆转录-聚合酶链反应检测各组水通道蛋白-1 (AQP1) mRNA表达水平。与单独使用药物相比,Ace和Cis联合治疗对Hep-2细胞的生长抑制和诱导凋亡具有协同作用。Ace/Cis联合应用可降低PCNA的表达,增加p53的表达。此外,联合处理降低了bcl-2/bax的比值,增加了caspase-3的表达,降低了AQP1的表达。这些结果表明,Ace和Cis联合使用可增强喉癌细胞的化疗敏感性。[3] |
| 体内研究 (In Vivo) |
乙酰唑胺 (40 mg/kg) 显着增强 MS-275 对神经母细胞瘤 (NB) SH-SY5Y 异种移植物肿瘤发生的抑制作用 [3]。乙酰唑酰胺钠 (40 mg/kg) 和/或 MS-275。乙酰唑胺 (40 mg/kg)、MS-275 和乙酰唑胺+MS-275 可减少 NB SH-SY5Y 异种移植物中的有丝分裂,并可治疗地减少 NB SH-SY5Y 异种移植物中的 HIF1-α 和 CAIX 表达 [3]。水肿标志物的表达[3]。乙酰唑胺钠(50 mg/kg;口服 3 天)可有效降低受感染口腔中的淋菌负荷 90% [6]。
淋球菌感染是世界范围内一项紧迫的公共卫生威胁,因为感染发病率不断增加,同时细菌对大多数抗生素的耐药性也在增加。这导致有效的治疗选择越来越少。毫无疑问,迫切需要开发新的、有效的抗淋球菌药物。为了发现新的抗淋球菌治疗药物,我们先前鉴定了乙酰唑胺,一种碳酸酐酶抑制剂,作为一种新的淋病奈瑟菌抑制剂。乙酰唑胺在0.5 ~ 4 μg/mL浓度范围内抑制淋病奈瑟菌的生长,表现出较强的体外抗淋球菌活性。本研究的目的是研究乙酰唑胺对淋病奈瑟菌生殖道感染小鼠模型的体内疗效。与用药物治疗的小鼠相比,乙酰唑胺在治疗三天后显著减少了感染小鼠阴道内90%的淋球菌负荷。这些结果表明,乙酰唑胺作为一种有希望的治疗选择值得进一步研究,以补充有限的抗淋球菌治疗药物。[6] |
| 细胞实验 |
AlamarBlue细胞毒性试验[2]
标准方案按描述执行。用AlamarBlue试剂观察Acetazolamide (AZ) /乙酰唑胺(Acetazolamide, AZ)、MS-275和AZ + MS-275处理的细胞与对照(DMSO- 0.2x10−4μ m)的存活率,每孔中加入占总体积10%的试剂4 h后进行荧光检测。采用SPECTRAmax Gemini分光光度计(激发波长540 nm;发射波长590nm)。 碘化丙啶细胞周期测定[2] 简单地说,用Acetazolamide (AZ)和/或MS-275处理过的2 × 106细胞用柠檬酸盐盐水提振,在80%的低温乙醇中固定48小时,然后将细胞制成颗粒,在2mg /mL的RNase A中重新悬浮5分钟。加入0.1 mg/mL的碘化丙啶溶液,在RT下孵育30分钟,细胞通过细胞过滤器过滤到5ml的聚苯乙烯管中。标记的细胞在BD FACSCAN流式细胞仪上分析。数据采用FlowJo软件上的Watson-Pragmatic模型进行拟合。 创面愈合试验[2] 将SH-SY5Y细胞接种于48孔板上的玻璃片上,以105个细胞/孔的密度在500 μl培养基中贴敷过夜,一式三份。井的底部用一条黑色直线作了定位。在90%的融合时,用200 μl的移液管尖端用标记向导划伤细胞单层。用培养基洗去松散的非贴壁细胞。在培养基中加入新鲜培养基,添加乙酰唑胺(AZ) (10 μM, 20 μM, 40 μM)和MS-275 (0.75 μM, 1.5 μM和3 μM),培养48 h。48 h后,用PBS洗涤细胞,4%多聚甲醛固定。在PBS中洗涤三次后,细胞用20%甲醇中1%结晶紫染色。在治疗0、48和72 h的时间点拍摄10倍原始放大倍数的相对比光显微图像。使用NIH Image J程序对迁移的细胞进行人工计数,以量化迁移到伤口区域的细胞数量。每个实验进行了三次,一式三份,并显示了一个代表性的分析。 对于药物处理,Hep-2细胞用Acetazolamide (ACE)(低浓度1×10−8 mol/l,这里称为Acetazolamide (ACE) l;或高浓度5×10−8 mol/l,这里称为AceH), Cis(1µg/ml)单独,或Cis与Ace (AceL+Cis,或AceH+Cis)联合作用48小时。用等体积的媒介物处理的细胞作为对照。所有实验中Ace浓度分别为1×10−8或5×10−8 mol/l。所有实验均以1µg/ml的浓度使用Cis。Cis和Ace均溶解于二甲亚砜(DMSO)中,然后加入PBS稀释至最终工作浓度。培养物中DMSO的终浓度不超过0.5%。用AceH单独、Cis单独或联合(AceH+Cis)或对照(对照)治疗HUVECs 48 h。[3] Annexin V凋亡测定[3] 凋亡细胞采用Annexin V-异硫氰酸荧光素(FITC)/碘化丙啶(PI)双染色,采用FITC-Annexin V凋亡检测试剂盒定量。对数生长期,将Hep-2细胞置于6孔板中。细胞分别用Acetazolamide (ACE)L、AceH、Cis、AceL+Cis、AceH+Cis或对照液处理48 h。然后用PBS洗涤细胞,用胰蛋白酶消化,用富钙HEPES缓冲液重悬。按照制造商的说明,用Annexin V-FITC和PI染色该悬液15分钟。最后用FlowJo软件对细胞进行分析。 |
| 动物实验 |
Xenograft studies for determining the in vivo efficacy of Acetazolamide (AZ) , MS-275, and Acetazolamide (AZ) + MS-275 combination [2]
For the in vivo xenograft study, 4–6 weeks-old female NOD/SCID mice were obtained from the animal facility. Subcutaneous xenograft tumors were developed by injecting SH-SY5Y cells (2 × 106) into the inguinal fat pad of NOD/SCID mice. When tumor diameter reached 0.5 cm, the mice were randomized into four groups (5 mice per group). The control and treatment groups received intraperitoneal injections of vehicle (PBS) or Acetazolamide (AZ) (40 mg/kg), MS-275 (20 mg/kg) or the combination, respectively, every day for 2 weeks. Experiments were terminated when tumor sizes exceeded 2 cm3 in volume or animals showed signs of morbidity. Tumor diameters were measured on a daily basis until termination. The long (D) and short diameters (d) were measured with calipers. Tumor volume (cm3) was calculated as V = 0.5 × D × d2. After euthanizing the mice, tumors were resected, weighed and fixed in 10% neutral-buffered formalin at room temperature and processed for histopathology. For the in vivo serial heterotransplantation analysis, 2x106 untreated and pretreated Acetazolamide (AZ) + MS-275 cells, manually and enzymatically dissociated from treated tumors, were injected subcutaneously to NOD/SCID mice. Growth rates were measured 2–3 times per week. On the 38th day, the animals were sacrificed, after which tumors were removed and weighed. Mice infection and treatment [6] Two days after pellet implantation (Day 0), the vagina of each mouse was rinsed with 50 mM HEPES (pH = 7.4), and each mouse was inoculated intravaginally with 20 μL of the prepared bacterial suspension of N. gonorrhoeae FA1090 (1.2 × 108 CFU/mL). Two days post-infection (Day +2), mice were randomly allocated into groups (n=10) and administered either Acetazolamide (50 mg/kg) or the vehicle (DMSO-Tween 80-PBS, 1:1:8) orally for three consecutive days. As a positive control, one group of mice was administered a single intraperitoneal dose of ceftriaxone (15 mg/kg in water). |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Carbonic anhydrase inhibitors are avidly bound by carbonic anhydrase and, accordingly, tissues rich in this enzyme will have higher concentrations of carbonic anhydrase inhibitors following systemic administration. /Carbonic Anhydrase Inhibitors/ Inhibitors of Carbonic Anhydrase. Drug: acetazolamide; Oral Absorption: nearly complete; Plasma Half-Life: 6-9 hours; and Route of Elimination: renal excretion of intact drug. /From table/ ACETAZOLAMIDE RELATED TO RESPONSE IN RABBIT; KIDNEY RESPONSE, MEASURED BY MONITORING URINE FLOW & NA ELIMINATION, URINE FLOW & NA ELIMINATION OCCUR IMMEDIATELY AFTER INJECTION CORRELATED WITH LOG DOSE. IV BOLUS INJECTIONS OF (14)C-LABELED, ACETAZOLAMIDE WERE MADE IN RABBITS. PLASMA, URINE, & WASHED RED BLOOD CELL CONCN WERE MEASURED, THE LATTER INDICATING BOUND DRUG. For more Absorption, Distribution and Excretion (Complete) data for ACETAZOLAMIDE (6 total), please visit the HSDB record page. Metabolism / Metabolites ACETAZOLAMIDE DOSE NOT UNDERGO METABOLIC ALTERATION. Biological Half-Life 3 to 9 hours Plasma half-life: 6-9 hours /From table/ |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Acetazolamide is of synthetic origin. Acetazolamide exists as a white to faintly yellowish-white, odorless crystalline powder. Acetazolamide is very slightly soluble in water and only slightly soluble in ethanol (approx 750 g/l); it is practically insoluble in ether and chloroform. Uses: Preoperative management of closed-angle glaucoma, or as an adjunct in the treatment of open-angle glaucoma. Abnormal retention of fluid: drug-induced edema, obesity, and congestive cardiac failure. Epilepsy Metabolic alkalemia. Periodic paralysis HUMAN EXPOSURE: Patients with either acute or chronic overdosage with acetazolamide may show signs of dehydration with thirst, lethargy, confusion, poor skin turgor, and prolonged capillary refill time, but may have a paradoxical continued diuresis. Electrolyte abnormalities include hyponatremia, hypokalemia, and a non-anion gap hyperchloremic metabolic acidosis in the more than mild ingestion which may lead to further deterioration in mental status, production of seizures, electrocardiographic abnormalities, and arrhythmias. Prior renal insufficiency will lead to increased toxicity at a given dose. There are idiosyncratic reactions producing bone marrow suppression with hepatic and renal insufficiency. Acetazolamide may also precipitate in the renal tubules producing calculi with renal colic. Hypokalemia may lead to muscular weakness, hyporeflexia, and hypochloremic metabolic alkalosis. In geriatric patients, a chronic metabolic acidosis may lead to a chronic compensatory hyperventilation which increases pulmonary vascular resistance and decreases left ventricular function. This can be especially significant in patients on concurrent beta-blocker or calcium channel blocker therapy. The ventricular fibrillation threshold may then be reduced. Cardiac arrhythmias may occur due to potassium deficiency. Abuse or overdose may result in pancreatitis. Hyperglycemia, hyperuricemia, and hyperlipidemia may occur with acute overdose or in chronic use or abuse. Hypersensitivity reactions such as rash, photosensitivity, thrombocytopenia, and pancreatitis are rare. Contraindications: Renal hyperchloremic acidosis. Addison's disease and all types of suprarenal gland failure. Conditions where there is known depletion of sodium and potassium (at least until this is treated). Long-term administration is contraindicated in patients with chronic closed angle-closure glaucoma. Known sensitivity to sulfonamides. Acetazolamide should not be used to alkalinize urine following salicylate overdose since it may worsen metabolic acidosis. Acetazolamide is well absorbed from the gastrointestinal tract. Acetazolamide is distributed throughout body tissues; it concentrates principally in erythrocytes, plasma and kidneys and to a lesser extent in liver, muscles, eyes and the central nervous system. Acetazolamide does not accumulate in tissues. The drug crosses the placenta in unknown quantities. Acetazolamide is tightly bound to carbonic anhydrase and high concentrations are present in tissues containing this enzyme such as erythrocytes and the renal cortex. There is a small amount of irreversible binding to red cells. It is 70 to 90% bound to plasma protein. Acetazolamide is not metabolized. Acetazolamide is excreted unchanged by the kidneys via tubular secretion and passive reabsorption. There is no evidence of enterohepatic circulation although small amounts of unchanged drug are eliminated in the bile. Acetazolamide is a carbonic anhydrase inhibitor. Acetazolamide reduces the formation of hydrogen and bicarbonate ions from carbon dioxide and water by noncompetitive, reversible inhibition of the enzyme carbonic anhydrase, thereby reducing the availability of these ions for active transport into secretions. One patient died of cholestatic jaundice after taking 13 g of acetazolamide in 26 days. In one patient, fatal bone marrow depression with leukopenia, thrombocytopenia, and anemia occurred after therapy with 500 mg of acetazolamide twice daily for 14 weeks. One case of renal failure (anuria) occurred in a patient after taking 500 mg of acetazolamide twice daily for 2 weeks. There have been no reports of congenital defects despite past widespread use though one women on 750 mg per day for glaucoma during the 1st and 2nd trimester had a baby with a sacrococcygeal teratoma but no causal link could be made. Teratogenicity tests in rats and mice showed the absence of fourth and fifth digits from the right forelimb in the offspring of rats and mice. There were no apparent lessions in the newborn of rabbits and monkeys. The drug crosses the placenta in unknown quantities. Potentially hazardous interactions: The effects of folic acid antagonists, oral hypoglycaemic agents and oral anticoagulants may be increased by acetazolamide. The urinary antiseptic effect of methenamine may be prevented by acetazolamide by keeping the urine alkaline. The alkalinization of the urine by acetazolamide can reduce the urinary excretion of many weak bases (including amphetamine, quinine, quinidine, and diethylcarbamazine) and thus enhance their pharmacological effects. In one patient taking phenytoin and acetazolamide drug-induced osteomalacia was reported. The more serious effects include blood disorders, skin toxicity and renal stone formation. Stevens-Johnson syndrome has not been reported. Symptomatic adverse effects: Flushing, thirst, headache, drowsiness, dizziness, fatigue, irritability, excitement, paresthesias, ataxia, hyperpnoa and gastrointestinal disturbances have all been reported (Dollery). Oral ingestion is the usual means of exposure. There is no appreciable dermal absorption. There is no significant absorption or local irritation. ANIMAL/PLANT STUDIES: Numerous animal studies have demonstrated that the toxicity of acetazolamide was very low in the species studied (mouse, dog, rat, monkey). International Programme on Chemical Safety; Poisons Information Monograph: Acetazolamide (PIM 005) (1995) Available from, as of May 16, 2008: https://www.inchem.org/pages/pims.html Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that maternal doses of acetazolamide up to 1000 mg daily produce low levels in milk and would not be expected to cause any adverse effects in breastfed infants. American, Canadian and French professional guidelines consider carbonic anhydrase inhibitors acceptable in breastfeeding. ◉ Effects in Breastfed Infants A breastfed (extent not stated) infant whose mother was taking sustained-release acetazolamide (Diamox Sequels) 500 mg twice daily exhibited no apparent adverse effects related to acetazolamide from day 6 to day 10 postpartum. A mother who was taking acetazolamide 250 mg orally twice daily as well as using 2 drops of timolol 0.5% eye drops daily and pilocarpine eye drops twice daily delivered a preterm infant at 36 weeks of gestation. The infant began 5 months of exclusive breastfeeding at 6 hours after birth. On day 2, the infant developed electrolyte abnormalities consisting of hypocalcemia, hypomagnesemia, and metabolic acidosis. The infant was treated with oral calcium gluconate and a single dose of intramuscular magnesium sulfate. Despite continued breastfeeding and maternal drug therapy, the infant's mild metabolic acidosis resolved on day 4 of life and the infant was gaining weight normally at 1, 3 and 8 months, but had mild hypotonicity. The authors considered the metabolic effects to be caused by transplacental passage of acetazolamide that resolved despite the infant being breastfed. The infant gained weight adequately during breastfeeding, but had some mild, residual hypertonicity of the lower limbs requiring physical therapy. Two women were receiving acetazolamide during pregnancy and postpartum for pseudotumor cerebri. Their infants had metabolic acidosis after birth. Both infants resolved their metabolic acidosis despite receiving maternal breastmilk. ◉ Effects on Lactation and Breastmilk A randomized, partially blinded trial compared acetazolamide 1 tablet (presumably 250 mg) by mouth daily, diethylstilbestrol 0.5 mg twice daily, placebo once daily and routine care in 243 mothers who wished to not breastfeed. Pain and breast fullness were assessed at least daily by blinded observers. In this dosage, acetazolamide was no more effective than placebo and somewhat less effective than diethylstilbestrol in relieving discomfort. Protein Binding 98% |
| 参考文献 | |
| 其他信息 |
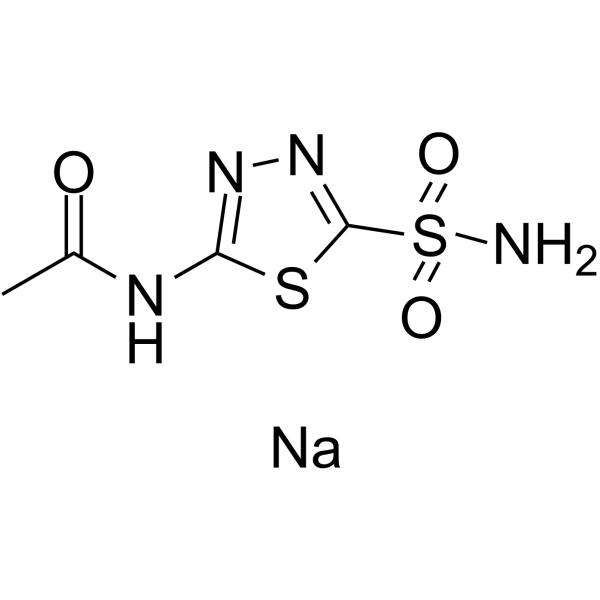
Acetazolamide sodium is an organic sodium salt. It contains an acetazolamide and an acetazolamide(1-).
Acetazolamide Sodium is the sodium salt of acetazolamide, a nonbacteriostatic sulfonamide derivative with diuretic and anticonvulsant properties. Acetazolamide is a potent inhibitor of carbonic anhydrase that plays an important role in the control of fluid secretion. Inhibition of this enzyme in the kidney results in a reduction in the availability of hydrogen ions for active transport in the renal tubule lumen, thereby leading to increased bicarbonate and cation excretion, and increased urinary volume. Reduced bicarbonate level in circulation induces reduction of intraocular pressure via osmotic mechanism. The anticonvulsant activity of acetazolamide may contribute to inhibition of carbonic anhydrase in the CNS, which decreases carbon dioxide tension in the pulmonary alveoli, thus increasing arterial oxygen tension. See also: Acetazolamide (has active moiety). Acetazolamide can cause developmental toxicity according to state or federal government labeling requirements. Acetazolamide appears as white to yellowish-white fine crystalline powder. No odor or taste. (NTP, 1992) Acetazolamide is a sulfonamide, a member of thiadiazoles and a monocarboxylic acid amide. It has a role as a diuretic, an anticonvulsant and an EC 4.2.1.1 (carbonic anhydrase) inhibitor. It is a conjugate acid of an acetazolamide(1-). It derives from a hydride of a 1,3,4-thiadiazole. One of the carbonic anhydrase inhibitors that is sometimes effective against absence seizures. It is sometimes useful also as an adjunct in the treatment of tonic-clonic, myoclonic, and atonic seizures, particularly in women whose seizures occur or are exacerbated at specific times in the menstrual cycle. However, its usefulness is transient often because of rapid development of tolerance. Its antiepileptic effect may be due to its inhibitory effect on brain carbonic anhydrase, which leads to an increased transneuronal chloride gradient, increased chloride current, and increased inhibition. (From Smith and Reynard, Textbook of Pharmacology, 1991, p337) Acetazolamide is a Carbonic Anhydrase Inhibitor. The mechanism of action of acetazolamide is as a Carbonic Anhydrase Inhibitor. Acetazolamide is a sulfonamide derivative with diuretic, antiglaucoma, and anticonvulsant properties. Acetazolamide is a non-competitive inhibitor of carbonic anhydrase, an enzyme found in cells in the proximal tube of the kidney, the eye, and glial cells. Inhibition of this enzyme in the kidney prevents excretion of hydrogen, leading to increased bicarbonate and cation excretion and increased urinary volume, which results in an alkaline diuresis. Acetazolamide reduces the concentration of bicarbonate, resulting in a decreased synthesis of aqueous humor in the eye, thereby lowering intraocular pressure. Although its mechanism of action is unknown, acetazolamide has anti-convulsant properties resulting from indirect effects secondary to metabolic acidosis or direct effects on neuronal transmission. Acetazolamide also produces respiratory stimulant effects in response to changes to both carbon dioxide and oxygen tension levels within the lungs. One of the CARBONIC ANHYDRASE INHIBITORS that is sometimes effective against absence seizures. It is sometimes useful also as an adjunct in the treatment of tonic-clonic, myoclonic, and atonic seizures, particularly in women whose seizures occur or are exacerbated at specific times in the menstrual cycle. However, its usefulness is transient often because of rapid development of tolerance. Its antiepileptic effect may be due to its inhibitory effect on brain carbonic anhydrase, which leads to an increased transneuronal chloride gradient, increased chloride current, and increased inhibition. (From Smith and Reynard, Textbook of Pharmacology, 1991, p337) See also: Acetazolamide Sodium (has salt form); Acetazolamide disodium (is active moiety of). Drug Indication For adjunctive treatment of: edema due to congestive heart failure; drug-induced edema; centrencephalic epilepsies; chronic simple (open-angle) glaucoma FDA Label Mechanism of Action The anticonvulsant activity of Acetazolamide may depend on a direct inhibition of carbonic anhydrase in the CNS, which decreases carbon dioxide tension in the pulmonary alveoli, thus increasing arterial oxygen tension. The diuretic effect depends on the inhibition of carbonic anhydrase, causing a reduction in the availability of hydrogen ions for active transport in the renal tubule lumen. This leads to alkaline urine and an increase in the excretion of bicarbonate, sodium, potassium, and water. Carbonic anhydrase inhibitors potently inhibit (IC50 for acetazolamide is 10 nM) both the membrane bound and cytoplasmic forms of carbonic anhydrase, resulting in nearly complete abolition of NaHCO3 reabsorption in the proximal tubule. /Carbonic Anhydrase Inhibitors/ Although the proximal tubule is the major site of action of carbonic anhydrase inhibitors, carbonic anhydrase also is involved in secretion of titratable acid in the collecting duct system (a process which involves a proton pump), and therefore the collecting duct system is a secondary site of action for this class of drugs. /Carbonic Anhydrase Inhibitors/ Acetazolamide frequently causes paresthesias and somnolence, suggesting an action of carbonic anhydrase inhibitors in the CNS. The efficacy of acetazolamide in epilepsy is in part due to the production of metabolic acidosis; however, direct actions of acetazolamide in the CNS also contribute to its anticonvulsant action. ... Inhibition of carbonic anhydrase decreases the rate of formation of aqueous humor and consequently reduce intraocular pressure. /Carbonic Anhydrase Inhibitors/ For more Mechanism of Action (Complete) data for ACETAZOLAMIDE (6 total), please visit the HSDB record page. Acetazolamide is the only carbonic anhydrase inhibitor with significant diuretic effects. It is readily absorbed and undergoes renal elimination by tubular secretion. Its administration is ordinarily marked by a brisk alkaline diuresis. Although carbonic anhydrase inhibitors are proximal tubular diuretics (where the bulk of sodium re-absorption occurs), their net diuretic effect is modest in that sodium re-absorption in more distal nephron segments offsets proximal sodium losses. Acetazolamide use is limited by both its transient action and the development of metabolic acidosis with extended administration. Acetazolamide can, however, correct the significant metabolic alkalosis which occasionally occurs with loop diuretic therapy.[3] The binding of antihypertensive acetazolamide with eleven nonsteroidal anti-inflammatory drugs (NSAIDs) was investigated at pH 3, 7 and 9.5 with the objective of monitoring their interactive pharmacokinetics during digestion and absorption in human body. The results of UV-Vis spectroscopy and cyclic voltammetry revealed two NSAIDs (acetaminophen and dichlofenic sodium) to interact with acetazolamide in stomach fluid conditions forming complexes of 1:1 and 1:2 stoichiometry. The complexation ratio was also verified by computational methods. The strong binding propensity of acetaminophen and dichlofenic sodium with acetazolamide prohibited their combined therapy. However, the poor binding affinity of aspirin and mefinamic acid suggested these drugs as preferred NSAIDs to be prescribed with acetazolamide.[4] |
| 分子式 |
C4H5N4O3S2-.NA+
|
|---|---|
| 分子量 |
244.2273
|
| 精确质量 |
243.97
|
| CAS号 |
1424-27-7
|
| 相关CAS号 |
Acetazolamide;59-66-5;Acetazolamide-d3;1189904-01-5
|
| PubChem CID |
13290219
|
| 外观&性状 |
White to off-white solid powder
|
| 熔点 |
258-259ºC (EFFERVESCENCE)
|
| LogP |
0.783
|
| tPSA |
142.87
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
14
|
| 分子复杂度/Complexity |
302
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
MRSXAJAOWWFZJJ-UHFFFAOYSA-M
|
| InChi Code |
InChI=1S/C4H6N4O3S2.Na/c1-2(9)6-3-7-8-4(12-3)13(5,10)11;/h1H3,(H3,5,6,7,9,10,11);/q;+1/p-1
|
| 化学名 |
sodium;(5-acetamido-1,3,4-thiadiazol-2-yl)sulfonylazanide
|
| 别名 |
Acetazolamide sodium; Sodium acetazolamide; 1424-27-7; Acetazolamide sodium salt; CHEBI:31163; 429ZT169UH; sodium;(5-acetamido-1,3,4-thiadiazol-2-yl)sulfonylazanide; N-(5-Sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide monosodium salt;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ≥ 100 mg/mL (~407.76 mM)
DMSO : ~100 mg/mL (~407.76 mM) |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0945 mL | 20.4725 mL | 40.9450 mL | |
| 5 mM | 0.8189 mL | 4.0945 mL | 8.1890 mL | |
| 10 mM | 0.4095 mL | 2.0473 mL | 4.0945 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01060969 | COMPLETED | Drug: Tadalafil and acetazolamide Drug: Acetazolamide |
Cerebral Edema Pulmonary Edema |
Sheba Medical Center | 2006-01 | Not Applicable |
| NCT05802849 | RECRUITING | Drug: Acetazolamide | Chronic Heart Failure | Samara State Medical University | 2023-05-01 | Phase 4 |
| NCT04887792 | RECRUITING | Drug: Acetazolamide Drug: Placebo |
Schizo Affective Disorder Schizophrenia |
Vishwajit Nimgaonkar, MD PhD | 2022-02-01 | Phase 1 Phase 2 |
| NCT04975269 | RECRUITING | Drug: Acetazolamide Drug: Placebo |
Idiopathic Normal Pressure Hydrocephalus (INPH) | Uppsala University Hospital | 2022-02-17 | Phase 2 |
| NCT01131377 | UNKNOWN STATUS | Drug: acetazolamide Drug: Saline |
Alkalosis, Metabolic Ventilator Weaning |
Asan Medical Center | 2010-05 | Not Applicable |