| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| Other Sizes |
| 靶点 |
mGluR7
|
|---|---|
| 体外研究 (In Vitro) |
在不改变基线谷氨酸释放的情况下,在 4-氨基吡啶处理之前将突触体与 AMN082 (1 μM) 预孵育 10 分钟,可有效抑制 4-氨基吡啶诱发的谷氨酸释放 [2]。
AMN082是一种选择性代谢型谷氨酸mGlu7受体激动剂,据报道具有抗抑郁活性。考虑到谷氨酸过度释放与抑郁症的发病机制有关,我们研究了N,N'-二苄基乙烷-1,2-二胺盐酸(AMN082)对大鼠脑皮质神经末梢谷氨酸释放的影响及其可能的机制。在本研究中,我们观察到AMN082抑制4-氨基吡啶诱发的谷氨酸释放,这一现象被谷氨酸mGlu7受体拮抗剂MMPIP阻断。此外,western blot分析和免疫细胞化学证实突触前代谢性谷氨酸mGlu7受体蛋白的存在。AMN-082通过螯合胞外Ca2+离子和囊泡转运蛋白抑制剂,阻止了4-氨基吡啶诱导谷氨酸释放的作用;然而,AMN082的作用不受谷氨酸转运蛋白抑制剂的影响。AMN082降低了4-氨基吡啶引起的突触体内Ca2+浓度升高,但没有改变突触体膜电位。在存在Cav2.2 (n型)和Cav2.1 (P/ q型)通道阻断剂、腺苷酸环化酶抑制剂和蛋白激酶A抑制剂的情况下,AMN082对4-氨基吡啶诱发的谷氨酸释放的作用明显降低。这些结果表明,AMN-082激活代谢性谷氨酸mGlu7受体可降低腺苷酸环化酶/蛋白激酶A的激活,从而减少Ca2+通过电压依赖性Ca2+通道进入并减少诱发的谷氨酸释放。另外,临床上有效的抗抑郁药氟西汀完全阻断了AMN082对谷氨酸释放的抑制作用,表明这两种化合物抑制谷氨酸从脑皮质神经末端释放的胞内机制是一致的。[2] 代谢性谷氨酸受体(mGluR)亚型(mGluR1至mGluR8)在中枢神经系统中作为神经传递的重要突触前和突触后调节因子。这些受体由两个结构域组成,一个是包含正位激动剂位点的细胞外区域,另一个是跨膜七螺旋结构域,参与G蛋白的激活和对几种新合成的药理学调节剂的识别。突触前受体mGluR7在家族中表现出最高的进化保守性,但没有已知的选择性药理工具。在这里,我们描述了一种mglur7选择性激动剂,N,N'-二苯并基乙烷-1,2-二胺盐酸(AMN082),它通过跨膜区域的变构位点直接激活受体信号。在转染表达mGluR7的哺乳动物细胞中,AMN082能有效抑制cAMP积累并刺激GTPgammaS结合(ec50值,64-290 nM),其激动剂效果与l -2-氨基-4-磷酸丁酸盐(L-AP4)相当,优于l -谷氨酸。AMN082(<或= 10微米)对其他mGluR亚型和部分嗜电离性glur没有明显的激活或抑制作用。嵌合受体研究将AMN082的结合位点定位在mGluR7的跨膜区域,我们证明这种变构激动剂对正构配体的效力几乎没有影响。本研究为3g家族蛋白偶联受体(具有mglur样结构)的七螺旋结构域介导的完全激动剂活性提供了证据,这可能会带来药物开发机会。[1] AMN082对克隆mGluR7的影响通过高通量随机筛选化学文库,鉴定出AMN082。AMN082的化学结构(图1)与已知的mGluR7配体完全无关,它们都来源于l-谷氨酸主链。该化合物在稳定表达人mGluR7b的CHO细胞中诱导了浓度依赖性抑制福斯柯林刺激的cAMP积累(图1a;EC50, 64±32 nM),与正位III组mGluR激动剂DL-AP4的饱和浓度相当(图1A,虚线)。在3 μM以下,AMN082对表达mglur2的CHO细胞没有影响(图1B)。通过刺激gtp - γ - 35s结合进一步评价AMN082的药理特性和作用机制。AMN082 (3 μM)相对于l-谷氨酸的最大激动剂活性(100%;图1 c)。AMN082的刺激作用与l-谷氨酸和DL-AP4几乎是相加的(分别为238±11%和336±19%),而DL-AP4加l-谷氨酸(都是在最大活性浓度下)产生的刺激作用比DL-AP4单独产生的刺激小(133±4%比216±12%,图1C)。因此,l-谷氨酸和DL-AP4可能在同一受体位点相互作用,完全激动剂DL-AP4的活性似乎被部分激动剂l-谷氨酸抑制。接下来,第三组mglr选择性拮抗剂MSOP和CPPG与AMN082在亚极大DL-AP4存在下的浓度-响应曲线进行测试(图1D): DL-AP4成分被拮抗剂完全消除,而AMN082刺激的gtp - γ 35s结合没有抑制作用。综上所述,图1c和D的数据表明,AMN082激活mGluR7信号很可能是通过与l-谷氨酸、DL-AP4、MSOP和CPPG等正构配体结合不同的位点来激活的。[1] AMN082、DL-AP4和l-谷氨酸的浓度响应曲线见图2A。AMN082的效价远高于正构配体DL-AP4和l-谷氨酸;EC50值(95%置信区间)为260 nM (200;360)、540 μm (440;700 μM (580;分别为850)。为了分析l-谷氨酸位点配体与AMN082之间的潜在协同作用,我们绘制了不同固定浓度的l-谷氨酸对AMN082的浓度-响应曲线,以及l-谷氨酸对固定浓度的AMN082的浓度-响应曲线(图2 B-D)。AMN082的EC50在140 ~ 290 nM之间变化,95%置信区间大部分重叠(图2 B和D)。同样,无论AMN082的添加浓度如何,l-谷氨酸的EC50始终在640 ~ 830 μM之间(图2 C和D)。为了研究AMN082与mGluR7的结合是否会影响配体对l-谷氨酸位点的结合亲和力,我们分析了10 nM [3H]LY341495(一种竞争性mGluR拮抗剂)与稳定表达mGluR7a的CHO细胞制备的膜的结合位移;在高达30 μM的AMN082中,该放射性配体未发生位移。相比之下,10 mM L-AP4、l-谷氨酸或L-SOP可100%消除特异性结合(数据未显示)。此外,我们在3 μM AMN082存在和不存在的情况下,分别用L-SOP、L-AP4和l-glutamate绘制了[3H]LY341495的位移曲线。AMN082的加入只引起三条曲线的轻微左移;计算得到的Ki值95%置信区间(括号内单位为μM)如下:L-SOP, 45 μM (39;50);L-SOP加3 μM AMN082, 29 μM (24;35);L-ap4, 193 μm (165;224);L-AP4加3 μM AMN082, 176 μM (144;216);l-谷氨酸,624 μM (446;870);和l-谷氨酸加3 μM AMN082, 524 μM (436;631)(数据未显示)。[1] AMN082直接与mGluR7的七螺旋区相互作用。接下来,我们打算将AMN082的结合位点定位到mGluR7蛋白的一个独立区域,并决定使用野生型mGluR7b和mGluR6构建体以及两种嵌合体:mGluR6/7b构建体包含mGluR6的n端胞外区域,mGluR7b的c端包含整个跨膜区域;mGluR7/6为反向嵌合体(见材料与方法及图3)。当使用gtp - γ - 35s结合刺激时,AMN082对mGluR6/7b和mGluR7/6嵌合体的活性分别与野生型mGluR7b和mGluR6非常相似:相对于DL-AP4的最大作用,AMN082对mglur7 /7b和mGluR6/7b的刺激作用为150-200%,而AMN082对mGluR6-和mGluR7/6表达膜的影响较小(相对于最大DL-AP4刺激作用为10-25%)(图3)。有趣的是,AMN082与DL-AP4联合对表达mglur7的细胞膜的刺激作用不仅仅是添加剂,而不是对表达mglur6 /7b的细胞膜的刺激作用(图3a和C)。此外,最初尝试使用缺失胞外结构域的截断突变体,但未观察到DL-AP4或AMN082的激活(数据未显示);不能排除它们的翻译蛋白被错误折叠或不正确地插入到膜中。[1] AMN082的选择性分析。在我们研究AMN082在所有8个已知的mglur和3个选定的嗜离子受体上的活性之前,我们通过放射性配位置换实验证实了1 μM的AMN082与30个不同的神经系统靶点没有显著的结合相互作用;该列表包括肾上腺素、多巴胺、GABA、组胺、乙酰胆碱、阿片类药物、血清素和P物质的受体,以及选定的神经递质再摄取位点(每个目标n = 2-4次测定;数据未显示)。图4显示了amno082对所有8个mglur和3个电离性glur的影响。当使用gtp - γ - 35s结合刺激时,3 μM和10 μM AMN082选择性地在mGluR7a和mGluR7b上表现出70-140%(相对于最大DL-AP4效应)的激活作用(图4 A和D)。在相同的实验设计下,AMN082(高达10 μM)对mGluR2-、mGluR3-、mGluR4-、mGluR6-或mglur8a表达细胞的膜几乎没有或没有刺激作用,并且在未转染的CHO细胞中也没有激活gtp - γ 35s结合(图4 B-D)。通过测量磷酸肌醇水解来确定AMN082是否激活I组mGluR亚型。AMN082在表达mGluR1b-或mglur5a的细胞中既没有表现出激动剂样活性,也没有表现出阳性的调节活性(图4D)。AMN082对两种NMDAR亚型和一种α-氨基-3-羟基-5-甲基-4-异氧唑丙酸(AMPA)受体亚型(NMDAR1a/2A、NMDAR1a/2B和GluR3)的功能激动剂和调节活性也被排除;图4 d);这个测试是通过细胞质钙的测定,使用稳定转染细胞系。此外,当使用上述功能受体检测格式时,高达10 μM的AMN082在任何测试的mGluR或离子型GluR亚型中均未显示出拮抗剂样作用(图4)[1]. |
| 体内研究 (In Vivo) |
AMN082(6 mg/kg;口服)以 mGluR7 依赖性方式使 mGluR7+/+ 小鼠(C57BL/6 遗传背景)的应激激素升高 [1]。 AMN082(1.25-5.0 mg/kg,腹腔注射;重复给药期间或可卡因或吗啡激发前每次注射可卡因或吗啡前 30 分钟)剂量依赖性地减弱可卡因或吗啡运动致敏的发展和表达 [3]。
AMN082的体内活性:mglur7依赖性应激激素的调节与所有已知的III类mGluRs的l-谷氨酸位点配体不同,AMN082在口服后很容易通过血脑屏障:口服10mg /kg的AMN082在大鼠和小鼠的脑组织中产生0.29 μmol/kg,口服14mg /kg的AMN082在口服1小时后分别产生0.62 μmol/kg(数据未显示)。 mGluR7在压力相关行为中的作用已被充分证明。因此,我们分析了口服AMN082对血清应激激素皮质酮和ACTH水平的影响。图5A显示,AMN082在野生型小鼠品系(C57BL/6)中以剂量依赖的方式增加血浆皮质酮。接下来,我们使用mGluR7缺陷小鼠(mGluR7-/-)和它们的野生型幼崽(mGluR7+/+)。同样,口服6mg /kg AMN082可引起mGluR7+/+动物血浆皮质酮增加约200%,但在mGluR7-/-小鼠中未观察到这种增加(图5B)。同样,在野生型动物口服AMN082 1小时后,血液中ACTH水平也增加到200%,但在mglur7缺陷小鼠中没有(图5C)。[1] 以往的研究表明,代谢性谷氨酸受体7 (mGluR7s)参与了药物成瘾。然而,这些受体在药物诱导的行为致敏中的作用尚不清楚。本研究的目的是确定全身注射选择性mGluR7变构激动剂AMN082是否能降低小鼠可卡因和吗啡诱导的多动和运动敏化的发生和表达,并影响小鼠对可卡因和吗啡刺激作用的相互交叉敏化。AMN082 (1.25-10.0 mg/kg, i.p)对小鼠的运动没有影响,也不影响可卡因或吗啡诱导的急性多动,但10 mg/kg的剂量抑制了两种药物的运动作用。反复暴露于可卡因或吗啡(10 mg/kg,每3天5次)在诱导致敏期间和分别在第17天或第20天给可卡因(10 mg/kg, i.p.)或吗啡(10 mg/kg, i.p.) 4天(可卡因)或7天(吗啡)戒断期后逐渐增加运动。低剂量AMN082预处理(1.25 ~ 5.0 mg/kg, i.p.p)在每次可卡因或吗啡注射前30分钟或在可卡因或吗啡刺激前进行预处理,剂量依赖性地减弱了可卡因或吗啡运动致敏的发展和表达。AMN082也抑制了这些药物之间的相互交叉致敏。在给予MMPIP (10 mg/kg, i.p)之前,选择性mGluR7拮抗剂逆转了AMN082对可卡因或吗啡致敏发展或表达的抑制作用。这些数据表明,AMN082通过涉及mGluR7s的机制减弱了可卡因和吗啡致敏的发展和表达,以及相互交叉致敏。因此,AMN082可能不仅在治疗可卡因或阿片类药物成瘾方面具有治疗意义,而且在治疗可卡因/阿片类药物滥用者方面也具有治疗意义。[3] AMN082治疗对急性可卡因或吗啡诱导的过度运动和基础运动活性的影响。 联合给药AMN082对可卡因和吗啡运动刺激作用致敏发展的影响。MMPIP对amno082效应的影响。 AMN082处理对可卡因和吗啡运动刺激作用致敏性表达的影响。MMPIP对amno082效应的影响。 AMN082处理对可卡因和吗啡相互运动交叉致敏表达的影响。[3] |
| 酶活实验 |
gtp - γ - 35s结合试验。[1]
用转染过的mGluR2-、mGluR3-、mGluR4-、mGluR6-、mGluR7a-、mGluR7b-、mGluR8a-、mGluR6/7b-和mglur7 /6表达细胞制备膜,按照Maj等人描述的方案进行gtp - γ 35s结合实验。 第二信使化验。[1] 如前所述,使用稳定表达单个mGluR亚型的CHO细胞系进行cAMP积累的测量。[3H]肌醇磷酸生成的测量方法参照Gasparini等人,钙的测量方法参照Maj等人。 [3H]LY341495结合试验。[1] 稳定表达mGluR7a的CHO细胞的膜组分(见上)在实验缓冲液(10 mM KH2PO4/100 mM KBr, pH 7.6)中稀释,用Polytron匀浆器短暂匀浆,在30°C下孵育10分钟。在96孔微滴板中配制检测混合物。最终体积为200 μl /孔的测定混合物的组成为:10 mM KH2PO4/100 mM KBr (pH 7.6),预处理膜蛋白50 μg,小麦胚芽凝集素闪烁邻近测定(WGA SPA)微球1.5 mg, 10 nM [3H]LY341495,适当浓度的试验化合物。在1 mM l-丝氨酸-磷酸(L-SOP)存在下测定非特异性结合。样品在室温下孵育60分钟(摇晃),然后在TopCount中计数。在棱镜程序中采用非线性回归对数据进行分析。利用Cheng和Prusoff方程将IC50值转换为Ki值。 |
| 细胞实验 |
谷氨酸释放[2]
通过在线荧光法测定谷氨酸释放(Nicholls和Sihra, 1986, Lin等,2015)。将颗粒状突触体重悬于含有16 μM牛血清白蛋白的HEPES缓冲介质中,并在Perkin-Elmer LS-55荧光计中于370C恒温搅拌皿中孵育。4-氨基吡啶(1 mM)诱导谷氨酸释放后,加入NADP+(2 mM)、谷氨酸脱氢酶(50单位/ml)和CaCl2(1.2 mM)。通过测量激发和发射波长分别为340和460 nm的NADPH荧光来监测释放的谷氨酸氧化脱胺导致NADP+的减少。每次实验结束后加入外源性谷氨酸标准品(5 nmol),根据标准品所产生的荧光变化计算每毫克突触体蛋白释放的谷氨酸(nmol/mg)纳摩尔。本文中引用的释放值和柱状图中描述的释放值表示5 min去极化后谷氨酸的累积释放水平,并以nmol/mg/5 min表示。数据以2-s为间隔进行累积,累积数据采用Lotus 1-2-3进行分析。 胞浆游离Ca2+浓度([Ca2+]C)在突触体群体[2] 用fura-2测定[Ca2+]C。将突触体(2 mg/ml)重悬于含有16 μM牛血清白蛋白的HEPES缓冲培养基中,在5 μM fura-2和0.1 mM CaCl2存在下,在37 °C搅拌试管中孵育30 min。fura-2装载后,将突触体制成颗粒,并在含有牛血清白蛋白的HEPES缓冲培养基中重悬。在Perkin-Elmer LS-55荧光仪中,将突触体悬浮液在含有1.2 mM CaCl2的恒温比色皿中搅拌,在激发波长340和380 nm(发射波长505 nm)处监测荧光。每隔2-s收集数据,并使用前面描述的公式计算[Ca2+]C (nM) (Grynkiewicz et al., 1985)。 Western blotting [2] Synaptosomes在Tris- cold缓冲溶液(50 mM Tris/HCl, 150 mM NaCl, 1% Triton X-100,蛋白酶抑制剂鸡尾酒,pH 7.4)中裂解,并用蛋白测定试剂盒定量蛋白质含量。然后,用SDS-PAGE分离蛋白,转移到硝化纤维素膜上,与以下兔单克隆抗体之一:代谢性谷氨酸mGlu7(1:500)和β-肌动蛋白(1:2000)在40℃下孵育过夜。洗涤后,用过氧化物酶偶联的山羊抗兔二抗(1:50,00)孵育1 h。免疫反应性检测采用增强化学发光和定量密度测定使用Syngene软件图中的值是至少三个独立实验的平均值。 免疫细胞化学[2]< br > 将突触体放置在直径为20 mm的聚赖氨酸包被盖上1 h,用4%多聚醛在0.1 M磷酸盐缓冲盐水中固定15 min,用0.2% Triton X-100磷酸盐缓冲盐水渗透5 min,用合适的抗谷氨酸1型囊泡转运体(1:20 00)或mGlu7受体(1:20 00)的一抗孵育24 h,用含有0.2% Triton X-100的0.9% NaCl的50 mm Tris缓冲液稀释。用含0.9% NaCl的Tris缓冲液洗涤后,用含0.9% NaCl的Tris缓冲液稀释的二抗孵育突触体1 h:山羊抗小鼠DyLight 549(红色;1:200)或山羊抗兔FITG(绿色;1:200)。在含0.9% NaCl的Tris缓冲液中冲洗几次后,用荧光液贴装盖片,用400倍放大倍率的直立荧光显微镜观察突触体,用CCD相机拍摄图像。通过计算至少三个不同的字段来分析每个覆盖。 |
| 动物实验 |
Animal/Disease Models: Male Swiss mice (20-25g) [3]
Doses: 1.25, 2.5, 5.0 mg/kg Route of Administration: intraperitoneal (ip) injection; on day 17 or day 20, cocaine (10 mg/kg) or Results of morphine (10 mg/kg) given 30 minutes before challenge: Dramatically attenuated the expression of cocaine-induced locomotor sensitization; attenuated morphine-induced sensitization. Animal Procedures, in Vivo AMN082 Administration. mGluR7-/- mice were generated as described from E14 (129/Ola) embryonic stem cells. All of the mice in the studies reported here carried wild-type or mutant mGluR7 alleles on a 14th-generation (F14) C57BL/6 genetic background. Age-matched groups of mGluR7-/- and mGluR7+/+ mice were generated as described. Male animals were used in all experiments. Food pellets and tap water were available ad libitum. Male mGluR7+/+ and littermate mGluR7-/- mice were injected orally (p.o.) with vehicle or 1-6 mg/kg AMN082. One hour later, mice (mGluR7-/- and mGluR7+/+ mice in a randomized order) were decapitated rapidly (within 30 sec after first touching the cage), and trunk blood was collected (n ≥ 9 per genotype). All animal experiments were subject to institutional review and conducted in accordance with the Veterinary Authority of Basel-Stadt. For i.p. injection, AMN082 and MMPIP were suspended in 0.5% methylcellulose, which was used as a vehicle. Both compounds were administered in a volume of 10 ml/kg (0.01 ml/g body weight). Fresh drug solutions were prepared on each day of the experiments. Drug injection times were determined based upon pilot studies and literature reports. [3] Effect of AMN082 treatment on the acute cocaine- or morphine-induced hyperlocomotion and basal locomotor activity [3] Mice (n = 6–10) were pretreated with a single injection of AMN082 (1.25, 2.5, 5.0 or 10 mg/kg, i.p.) or vehicle 30 min before injection of cocaine (10 mg/kg, i.p.) or morphine (10 mg/kg, i.p.). Locomotor activity was recorded for 30 or 60 min, respectively, immediately after cocaine/morphine administration. Furthermore, an influence of AMN082 (1.25, 2.5, 5.0 and 10 mg/kg, i.p.) alone on the locomotor activity of naive mice was examined. Effect of co-administration of AMN082 on the development of sensitization to the locomotor stimulant effect of cocaine and morphine. Influence of MMPIP on the AMN082 effect [3] To investigate the influence of AMN082 on the development of sensitization to the locomotor stimulant effect of cocaine, the mice (n = 7–9) were pretreated with AMN082 (1.2, 2.5 or 5.0 mg/kg, i.p.) or vehicle, 30 min before cocaine (10 mg/kg, i.p.) or saline (control group) injection on the first day (day 1) of the experiment. The mice were directly placed in the test apparatus and locomotor activity was measured for the following 30 min. The administration procedure was repeated on days 4, 7, 10, and 13, and locomotor activity measurements were performed as described above. To determine whether mGluR7s are involved in the AMN082 effect on the development of cocaine sensitization, the cocaine-treated group was pretreated with MMPIP (10 mg/kg, i.p.), an allosteric mGluR7-selective antagonist, 30 min prior to AMN082 (5.0 mg/kg, i.p.) injection. Control mice were injected with saline and vehicle. Following a withdrawal period of 4 days (day 17), all groups of mice were challenged with cocaine (10 mg/kg, i.p.) without AMN082 and/or MMPIP administration and the locomotor activity was measured for 30 min. To investigate the influence of AMN082 on the development of sensitization to the locomotor stimulant effect of morphine, the mice (n = 6–10) were pretreated with AMN082 (1.25, 2.5 or 5.0 mg/kg, i.p.) or vehicle, 30 min before each morphine (10 mg/kg, i.p.) or saline (control group) injection every 3 days, for five times (on the 1st, 4th, 7th, 10th and 13th days). Immediately after morphine administration, locomotor activity was measured for 60 min. To determine whether the mGluR7s are involved in the AMN082 effect on the induction of morphine locomotor sensitization, the morphine-treated group was pretreated with MMPIP (10 mg/kg, i.p.), 30 min prior to AMN082 (5.0 mg/kg, i.p.) injection. Control mice were injected with saline and vehicle. Following a withdrawal period of 7 days (day 20), all groups of mice received a challenge dose of morphine (10 mg/kg, i.p.) without AMN082 and/or MMPIP administration and locomotor activity was measured for 60 min. Effect of AMN082 treatment on the expression of sensitization to the locomotor stimulant effect of cocaine and morphine. Influence of MMPIP on the AMN082 effect [3] In order to test the effect of AMN082 on the expression of sensitization to the locomotor stimulant effect of cocaine, separate groups of mice (n = 8–10) were sensitized to cocaine as described above. On the test day (17th day of experiment), mice were treated with AMN082 (1.25, 2.5 or 5.0 mg/kg i.p.) or vehicle 30 min before the cocaine challenge (10 mg/kg, i.p.), and the locomotor activity was measured for 30 min. To determine whether mGluR7s are involved in this AMN082 effect on the expression of cocaine sensitization, the cocaine-sensitized mice, before cocaine challenge (10 mg/kg, i.p.), were pretreated with MMPIP (10 mg/kg, i.p.), 30 min prior to AMN082 (5.0 mg/kg, i.p.) injection. Immediately after cocaine challenge, locomotor activity was measured for 30 min. To evaluate an influence of the mGluR7 agonist on the expression of morphine-induced locomotor sensitization, separate groups of mice (n = 8–10) were sensitized to morphine, as described above. On the test day (20th day of experiment), 30 min prior to the challenge dose of morphine (10 mg/kg, i.p.) mice were treated with AMN082 (1.25, 2.5 or 5.0 mg/kg, i.p.) or vehicle and locomotor activity was recorded for 60 min. To determine whether the mGluR7s are involved in the effect of AMN082 on the expression of morphine sensitization, the morphine sensitized mice, before morphine challenge (10 mg/kg, i.p.), were pretreated with MMPIP (10 mg/kg, i.p.), 30 min before AMN082 (5.0 mg/kg) injection. Immediately after morphine challenge, locomotor activity was measured for 60 min. Effect of AMN082 on the expression of reciprocal locomotor cross-sensitization between cocaine and morphine [3] The induction of cocaine sensitization was performed according to the method described above. Following a period of 4 days without treatment (17th day of experiment), the cocaine-sensitized and saline-treated mice (n = 7–10) were pretreated with AMN082 (2.5 or 5.0 mg/kg, i.p.) or vehicle, 30 min before the challenge with morphine (10 mg/kg, i.p.). Locomotor activity was measured for 30 min, immediately after morphine challenge. The induction of morphine sensitization was performed according to the method described above. Following a period of 7 days without treatment (20th day of experiment) the morphine-sensitized and saline-treated mice (n = 9–10) were pretreated with AMN082 (2.5 or 5.0 mg/kg, i.p.) or vehicle, 30 min before the challenge with cocaine (10 mg/kg, i.p.). Locomotor activity was measured for 60 min, immediately after the cocaine challenge. |
| 参考文献 |
|
| 其他信息 |
AMN082 dihydrochloride is a hydrochloride obtained by combining N,N'-bis(diphenylmethyl)ethane-1,2-diamine with two molar equivalent of hydrochloric acid. It has a role as a metabotropic glutamate receptor agonist, a geroprotector and a neuroprotective agent. It contains a N,N'-bis(diphenylmethyl)ethane-1,2-diamine(2+).
In these studies, we present the first selective mGluR7 agonist, AMN082. This compound acts via a previously undescribed site, is orally active, and selectively modulates the in vivo levels of two stress hormones, corticosterone and ACTH in wild-type, but not in mGluR7-deficient, mice, substantiating further a role for mGluR7 in stress physiology. AMN082 elicits a full agonist response, comparable with L-AP4, in the absence of glutamate site ligands. Our data with chimeric receptors strongly suggest the localization of an allosteric agonist site within the transmembrane domain of mGluR7. Definitive proof of this allosteric site awaits the development of an appropriate radioligand, which will enable determination of physicochemical binding characteristics, such as Kd, Kon, and Koff. However, our chimeric receptor data, together with the absence of AMN082 activity at other G protein-coupled mGluRs expressed in identical host cells, as were used for mGluR7 functional studies, strongly argue that the agonist activity of AMN082 results from a direct compound interaction with the mGluR7 protein. In particular, this mGluR7-dependent agonist activity is seen in both the G protein assay (GTPγS binding) and in second-messenger determinations (cAMP accumulation), which drastically reduces the possibility of AMN082 interacting with components of the intracellular signaling cascades. In conclusion, we have identified a selective mGluR7 agonist, AMN082, that acts via an allosteric site and can serve as an invaluable tool for further unraveling the role of mGluR7 in stress-related CNS disorders. [1] AMN082 is a selective metabotropic glutamate mGlu7 receptor agonist reported to exhibit antidepressant activity. Considering that excessive glutamate release is involved in the pathogenesis of depression, the effect of N,N'-dibenzyhydryl-ethane-1,2-diamine dihydrochloride (AMN082) on glutamate release in rat cerebrocortical nerve terminals and the possible underlying mechanism were investigated. In this study, we observed here that AMN082 inhibited 4-aminopyridine-evoked glutamate release and this phenomenon was blocked by the metabotropic glutamate mGlu7 receptor antagonist MMPIP. Moreover, western blot analysis and immunocytochemistry confirmed the presence of presynaptic metabotropic glutamate mGlu7 receptor proteins. The effect of AMN082 on the 4-aminopyridine-evoked release of glutamate was prevented by chelating the extracellular Ca2+ ions and the vesicular transporter inhibitor; however, the effect of AMN082 was unaffected by the glutamate transporter inhibitor. AMN082 reduced the elevation of 4-aminopyridine-evoked intrasynaptosomal Ca2+ concentration, but did not alter the synaptosomal membrane potential. In the presence of the Cav2.2 (N-type) and Cav2.1 (P/Q-type) channel blocker, the adenylate cyclase inhibitor, and the protein kinase A inhibitor, the action of AMN082 on the 4-aminopyridine-evoked glutamate release was markedly reduced. These results suggest that the activation of the metabotropic glutamate mGlu7 receptors by AMN082 reduces adenylate cyclase/protein kinase A activation, which subsequently reduces the entry of Ca2+ through voltage-dependent Ca2+ channels and decreases evoked glutamate release. Additionally, fluoxetine, a clinically effective antidepressant, completely occluded the inhibitory effect of AMN082 on glutamate release, thus indicating the existence of a common intracellular mechanism for these two compounds to inhibit glutamate release from the cerebrocortical nerve terminals. [2] Finally, we propose that suppression of the adenylate cyclase/cyclic adenosine monophosphate/protein kinase A pathway is involved in the inhibitory action of AMN082 on the 4-aminopyridine-evoked glutamate release from cerebrocortical nerve terminals. This suggestion is based on the following results: (1) AMN082 inhibited the facilitatory effect of forskolin on the 4-aminopyridine-evoked glutamate release; and (2) the inhibitory effects of AMN082 on the 4-aminopyridine-evoked glutamate release were prevented by the adenylate cyclase inhibitor MDL12330A and protein kinase A inhibitor H89. Our findings are consistent with a report by Summa et al. (2013), which demonstrated that AMN082 acts at the metabotropic glutamate mGlu7 receptors present in the hippocampal nerve terminals and reduces the adenylate cyclase/cyclic adenosine monophosphate production, which subsequently inhibits the evoked γ-aminobutyric acid release. Previous studies have found that metabotropic glutamate mGlu7 receptors are present at the presynaptic level, and their activation inhibits glutamate release through a reduction of the Gi/o protein-coupled adenylate cyclase/protein kinase A pathway (Millán et al., 2002). Our data suggest that the activation of metabotropic glutamate mGlu7 receptors by AMN082 reduces adenylate cyclase activation and protein kinase A activity, which subsequently reduces the Ca2+ influx through the Cav2.2 (N-type) and Cav2.1 (P/Q-type) Ca2+ channels to cause a reduction in evoked glutamate release from cerebrocortical nerve terminals. In conclusion, the current study provides evidence that the metabotropic glutamate mGlu7 receptor agonist, AMN082, exerts an inhibitory effect on glutamate release from rat cerebrocortical nerve terminals. Although the functional role of AMN082-inhibited glutamate release explored here is not clear, such an effect is a potentially crucial mechanism for the treatment of brain disorders, such as depression, which are associated with excessive glutamate release (Hashimoto et al., 2007). [2] Published data indicated that AMN082 did not affect extracellular glutamate or GABA release in the VP in cocaine-abstinent rats (Li et al., 2010), suggesting that glutamate and GABA-ergic transmission is not involved in AMN082-induced inhibition of cocaine-primed relapse. This suggests that different mechanisms appear to underlie antagonism by AMN082 of intravenous cocaine self-administration (by VP GABA-ergic mechanism) and of cocaine-induced reinstatement of drug-seeking behavior (by NAc glutamate mechanism). However, it has been indicated that the VP is involved in the expression of morphine locomotor sensitization after long- (3 weeks) but not short-term (3 days) of morphine withdrawal (Mickiewicz et al., 2009). Thus, more studies are required to fully explicate the manner, in which AMN082 inhibits cocaine and morphine sensitization. The present study demonstrates, for the first time, that the selective mGluR7 allosteric agonist AMN082 inhibits the development and expression of cocaine- and morphine-induced sensitization and cross-sensitization between these drugs at low doses that did not disturb the acute locomotor hyperactivity induced by these drugs. These findings suggest an important role of mGluR7 in the sensitization phenomenon because MMPIP, an allosteric mGluR7-selective antagonist, blocked these effects of AMN082. Thus, our studies suggest that mGluR7s are involved in neuroadaptive processes associated with cocaine and morphine addiction. Finally, our findings indicate that mGluR7 agonists can be effective in preventing cocaine/morphine-dependent individuals from relapsing to these drugs. [3] |
| 分子式 |
C28H29CLN2
|
|---|---|
| 分子量 |
428.996266126633
|
| 精确质量 |
464.179
|
| 元素分析 |
C, 72.25; H, 6.50; Cl, 15.23; N, 6.02
|
| CAS号 |
97075-46-2
|
| 相关CAS号 |
AMN082 free base;83027-13-8
|
| PubChem CID |
11698390
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
8.13
|
| tPSA |
24.06
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
362
|
| 定义原子立体中心数目 |
0
|
| SMILES |
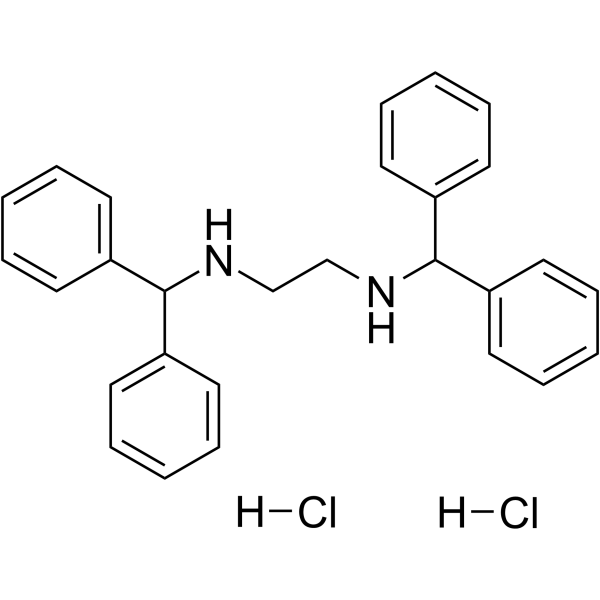
Cl.C1C=CC(C(C2C=CC=CC=2)NCCNC(C2C=CC=CC=2)C2C=CC=CC=2)=CC=1
|
| InChi Key |
YRQCDCNQANSUPB-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C28H28N2.2ClH/c1-5-13-23(14-6-1)27(24-15-7-2-8-16-24)29-21-22-30-28(25-17-9-3-10-18-25)26-19-11-4-12-20-26;;/h1-20,27-30H,21-22H2;2*1H
|
| 化学名 |
N,N'-dibenzhydrylethane-1,2-diamine;dihydrochloride
|
| 别名 |
AMN082 DIHYDROCHLORIDE; 97075-46-2; AMN082; AMN 082 dihydrochloride; 83027-13-8; N,N'-Dibenzhydrylethane-1,2-diamine dihydrochloride; AMN 082 (Free base); N1,N2-Dibenzhydrylethane-1,2-diamine dihydrochloride;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~33.33 mg/mL (~71.61 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3310 mL | 11.6550 mL | 23.3100 mL | |
| 5 mM | 0.4662 mL | 2.3310 mL | 4.6620 mL | |
| 10 mM | 0.2331 mL | 1.1655 mL | 2.3310 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|