| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
| 靶点 |
Estrogen receptor
|
|---|---|
| 体外研究 (In Vitro) |
ARV-471是一种雌激素受体(ER)α-PROTAC,是一种杂双功能分子,促进雌激素受体α与细胞内E3连接酶复合物之间的相互作用,导致雌激素受体通过蛋白酶体泛素化并随后降解。ARV-471以~2nM的半最大降解浓度(DC50)强力降解ER-阳性乳腺癌症细胞系中的ER。PROTAC介导的ER降解降低了经典调节的ER靶基因(PR、GREB1、TFF)的表达,并抑制了ER依赖性细胞系(MCF7、T47D)的细胞增殖。此外,ARV-471可降解临床相关的ESR1变体(Y537S和D538G),并抑制表达这些变体的细胞系的生长[2]。
|
| 体内研究 (In Vivo) |
在未成熟的大鼠子宫营养模型中,ARV-471会降解大鼠子宫ER,并且没有表现出激动剂活性。每日口服单药ARV-471(3、10和30 mpk)会导致雌二醇依赖性MCF7异种移植物的肿瘤体积显著缩小,并在研究结束时伴随肿瘤ER蛋白减少>90%。此外,当CDK4/6抑制剂在MCF7模型中与ARV-471联合使用时,观察到更明显的肿瘤生长抑制(约130%TGI),同时ER蛋白水平显著降低。在ESR1 Y537S,激素非依赖性患者来源的异种移植物模型中,10 mpk的ARV-471完全抑制了生长,也降低了突变ER蛋白水平。综上所述,ARV-471的临床前数据支持其作为同类最佳口服ER PROTAC降解剂的持续发展[2]。
|
| 细胞实验 |
蛋白质印迹程序[Clin Cancer Res. 2024 Aug 15;30(16):3549-3563.]
所有细胞系和子宫或异种移植物肿瘤组织在RIPA裂解缓冲液和Halt蛋白酶抑制剂中裂解/均质化。降解试验中的ER蛋白水平通过标准蛋白质印迹、细胞内蛋白质或在WES或JESS仪器上进行的数字蛋白质分析进行测量。完整方法见补充扩展方法。
细胞生长抑制试验[Clin Cancer Res. 2024 Aug 15;30(16):3549-3563.] 除非另有说明,否则在96孔板上以2000个细胞/孔的速度进行细胞生长抑制研究,使用3倍连续稀释的8点DRCs。在第5天,使用cell Titer Glo测量细胞存活率,并使用GraphPad分析CTG数据。活细胞成像增殖和剂量基质药物组合测定在补充扩展方法中进行了描述。 |
| 动物实验 |
Immature rat uterotrophic assay [Clin Cancer Res. 2024 Aug 15;30(16):3549-3563.]
This model was conducted as previously described using immature female rats younger than postnatal day (PND) 30. Sprague–Dawley (SD) rats at PND 18 were dosed with 30 mg/kg vepdegestrant or 10 mg/kg AZD-9496 in vehicle of PEG400/2% Tween80, by oral gavage (per os, po) once daily for 3 days (qdx3), or a single subcutaneous (sc) dose of 100 mg/kg fulvestrant in a vehicle of 10% w/v ethanol, 10% w/v benzyl alcohol, and 15% w/v benzyl benzoate, made up to 100% w/v with castor oil (EBB/castor oil). Five animals were used per arm. Animals were euthanized and tissues harvested 24-hours post-last dose or on day 4 for fulvestrant/sc arms. Uterine weights were measured, flash frozen in liquid nitrogen, and stored at −80°C. ER levels were determined by western blot. MCF7 orthotopic xenograft model[Clin Cancer Res. 2024 Aug 15;30(16):3549-3563.] Eight- to 10-week-old female NOD/SCID mice were surgically implanted with a 0.36 mg 90-day release 17β-estradiol pellet subcutaneously. One to 2 days later, each mouse was injected with 5 × 106/200 µL MCF7 cells (ATCC) into one mammary fat pad. Cells were prepared in a 50/50 RPMI-1640 phenol red-free media/Corning Matrigel Membrane Matrix mix at 25 × 106 cells/mL. Dosing was initiated once tumors reached an average of 200 mm3. When oral combinations were dosed, vepdegestrant was dosed first and the second agent 30 to 60 minutes later. All oral agents (vepdegestrant, palbociclib, abemaciclib, ribociclib, inavolisib, alpelisib, and everolimus) were dosed at 5 mL/kg volume once daily for 28 days (qdx28) unless otherwise stated. Fulvestrant sc was dosed at 4 mL/kg twice per week (biw) for 2 weeks plus once per week (qw) for 2 weeks (biwx2, qwx2). Vehicles for the various compounds dosed in vivo are listed in Supplementary Table S5. Tumor volumes were measured twice per week in efficacy studies and calculated using (width2 × length)/2, in which all measurements are in millimeters (mm), and the tumor volume is in mm3. Body weights were recorded twice per week. In some drug combinatorial efficacy studies, some single-day dosing holidays (small black arrows in Fig. 6D and andE)E) were implemented on all arms if any body weight loss approached 10%. At study termination, mice were euthanized 18 hours post-last dose, and harvested tissue was snap-frozen on dry ice. TGI was calculated as follows, with tumor volume being expressed in mm3. |
| 参考文献 | |
| 其他信息 |
Estrogen receptor alfa (ERα) is expressed in approximate 70% of breast cancer (BC) which is the most common malignancy in women worldwide. To date, the foremost intervention in the treatment of ER positive (ER+) BC is still the endocrine therapy. However, resistance to endocrine therapies remains a major hurdle in the long-term management of ER + BC. Although the mechanisms underlying endocrine resistance are complex, cumulative evidence revealed that ERα still plays a critical role in driving BC tumor cells to grow in resistance state. Fulvestrant, a selective estrogen receptor degrader (SERD), has moved to first line therapy for metastatic ER + BC, suggesting that removing ERα would be a useful strategy to overcome endocrine resistance. Proteolysis-Targeting Chimera (PROTAC) technology, an emerging paradigm for protein degradation, has the potential to eliminate both wild type and mutant ERα in breast cancer cells. Excitingly, ARV-471, an ERα-targeted PROTAC developed by Arvinas, has been in phase 1 clinical trials. In this review, we will summarize recent progress of ER-targeting PROTACs from publications and patents along with their therapeutic opportunities for the treatment of endocrine-resistant BC.[1]
Vepdegestrant is an orally available hetero-bifunctional molecule and selective estrogen receptor (ER) alpha-targeted protein degrader, using the proteolysis targeting chimera (PROTAC) technology, with potential antineoplastic activity. Vepdegestrant is composed of an ER alpha ligand attached to an E3 ligase recognition moiety. Upon oral administration,vepdegestrant targets and binds to the ER ligand binding domain on ER alpha. E3 ligase is recruited to the ER by the E3 ligase recognition moiety and ER alpha is tagged by ubiquitin. This causes ubiquitination and degradation of ER alpha by the proteasome. This decreases ER alpha protein levels, decreases the expression of ER alpha-target genes and halts ER-mediated signaling. This results in an inhibition of proliferation in ER alpha-overexpressing tumor cells. In addition, the degradation of the ER alpha protein releases the ARV-471 and can bind to additional ER alpha target proteins. ER alpha is overexpressed in a variety of cancers and plays a key role in cancer cell proliferation. |
| 分子式 |
C45H49N5O4
|
|---|---|
| 分子量 |
723.901671171188
|
| 精确质量 |
723.378
|
| 元素分析 |
C, 74.66; H, 6.82; N, 9.67; O, 8.84
|
| CAS号 |
2229711-08-2
|
| 相关CAS号 |
2229711-68-4;
|
| PubChem CID |
134579471
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
6.4
|
| tPSA |
96.4Ų
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
54
|
| 分子复杂度/Complexity |
1310
|
| 定义原子立体中心数目 |
2
|
| SMILES |
C1CC2=C(C=CC(=C2)O)[C@H]([C@H]1C3=CC=CC=C3)C4=CC=C(C=C4)N5CCC(CC5)CN6CCN(CC6)C7=CC8=C(C=C7)C(=O)N(C8)C9CCC(=O)NC9=O
|
| InChi Key |
TZZDVPMABRWKIZ-MFTLXVFQSA-N
|
| InChi Code |
InChI=1S/C45H49N5O4/c51-37-12-15-39-33(27-37)8-13-38(31-4-2-1-3-5-31)43(39)32-6-9-35(10-7-32)48-20-18-30(19-21-48)28-47-22-24-49(25-23-47)36-11-14-40-34(26-36)29-50(45(40)54)41-16-17-42(52)46-44(41)53/h1-7,9-12,14-15,26-27,30,38,41,43,51H,8,13,16-25,28-29H2,(H,46,52,53)/t38-,41?,43+/m1/s1
|
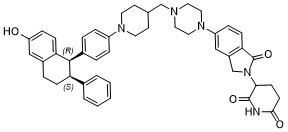
| 化学名 |
3-(5-(4-((1-(4-((1R,2S)-6-hydroxy-2-phenyl-1,2,3,4-tetrahydronaphthalen-1-yl)phenyl)piperidin-4-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione
|
| 别名 |
ARV471; (Rac)-Vepdegestrant; 2229711-08-2; 3-(5-(4-((1-(4-((1R,2S)-6-Hydroxy-2-phenyl-1,2,3,4-tetrahydronaphthalen-1-yl)phenyl)piperidin-4-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dione; ARV471; vepdegrestrant; EX-A5021; starbld0007729; SCHEMBL20231167; ARV 471; ARV-471
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 补充信息 |
psy_string | [
"Molecular Formula: C45H49N5O4",
" Exact Mass: 723.3785",
" Molecular Weight: 723.92Synonym: ARV471",
" ARV 471",
" ARV-471",
"Chemical Name:3-(5-(4-((1-(4-((1R,2S)-6-hydroxy-2-phenyl-1,2,3,4-tetrahydronaphthalen-1-yl)phenyl)piperidin-4-yl)methyl)piperazin-1-yl)-1-oxoisoindolin-2-yl)piperidine-2,6-dioneInChi Key:TZZDVPMABRWKIZ-MFTLXVFQSA-NInChi Code:InChI=1S/C45H49N5O4/c51-37-12-15-39-33(27-37)8-13-38(31-4-2-1-3-5-31)43(39)32-6-9-35(10-7-32)48-20-18-30(19-21-48)28-47-22-24-49(25-23-47)36-11-14-40-34(26-36)29-50(45(40)54)41-16-17-42(52)46-44(41)53/h1-7,9-12,14-15,26-27,30,38,41,43,51H,8,13,16-25,28-29H2,(H,46,52,53)/t38-,41?,43+/m1/s1SMILES Code:OC1=CC=C2C(CC[](C3=CC=CC=C3)[C@@H]2C4=CC=C(N5CCC(CN6CCN(C7=CC(CN(C8CCC(NC8=O)=O)C9=O)=C9C=C7)CC6)CC5)C=C4)=C1"
]
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3814 mL | 6.9070 mL | 13.8141 mL | |
| 5 mM | 0.2763 mL | 1.3814 mL | 2.7628 mL | |
| 10 mM | 0.1381 mL | 0.6907 mL | 1.3814 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。