| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
mGlu2 receptor
|
|---|---|
| 体外研究 (In Vitro) |
体外电生理试验[1]
mGlu2/3受体激动剂DCG-IV对海马CA1区锥体细胞中的fEPSPs具有抑制作用。AZD8418和AZD8529增强了DCG-IV对突触传递的抑制作用,EC50值分别为0.86和1.4μM(图2a)。AZD8418产生的最大增强作用(79.9±4.4)大于AZD8529(61.4±4.3,p<0.05)。mGlu2/3受体拮抗剂LY341495(1μM)有效阻断了DCG-IV单独(10 nM)或DCG-IV与AZD8529(10μM)或AZD8418(10μM)联合诱导的fEPSPs抑制作用;图2b)。 |
| 体内研究 (In Vivo) |
AZD8418(0.37、1.12、3.73、7.46和14.92 mg/kg)和AZD8529(1.75、5.83、17.5和58.3 mg/kg)的急性治疗抑制了尼古丁的自我给药,对食物维持反应没有影响。AZD8418的慢性治疗减弱了尼古丁的自我给药,但对这种作用的耐受性迅速发展。慢性AZD8529给药对尼古丁自我给药的抑制作用在整个14天的治疗过程中持续存在。任何一种PAM的慢性治疗都会抑制食物的自我给药。AZD8418(急性)和AZD8529(急性和亚慢性)阻断了线索诱导的尼古丁和食物寻求行为的恢复。
结论:这些发现表明,mGlu2受体在自我给药尼古丁的增强特性以及之前与尼古丁给药相关的线索的动机影响(即线索诱导的尼古丁寻求行为的恢复)中起着重要作用。因此,mGlu2-PAM可能是帮助人们戒烟和防止复发的有用药物[1]。
|
| 细胞实验 |
切片记录[1]
将切片浸入切片室中,以1-2ml/min的流速浸泡在32°C aCSF中。用铂丝制成的砝码将切片固定到位。用连接到隔离脉冲刺激器(型号2100)的单极钨电极(型号575300,0.5-1mΩ)刺激辐射层中的Schaeffer侧支纤维。用从硼硅酸盐玻璃(型号TW150-4)中拔出并填充2mM NaCl的电极记录CA1层锥体细胞的细胞外群体尖峰。用每30秒一次的10毫秒脉冲刺激切片。建立基线反应(最大值的50-70%),然后记录大约15分钟的控制期。在控制期后,将化合物浴敷40分钟或直至达到稳态响应。 |
| 动物实验 |
Experimental design [1]
Experiment 1: effects of acute AZD8418 and AZD8529 treatment on nicotine and food self-administration [1] After establishing stable nicotine or food self-administration (<20 % variability in responding over three consecutive days), the effects of acute AZD8418 (0, 0.37, 1.12, 3.73, 7.46, and 14.92 mg/kg) and AZD8529 (0, 1.75, 5.83, 17.5, and 58.3 mg/kg) treatment on nicotine and food self-administration were assessed using a within-subjects Latin square design. Four groups of naive rats were used to examine the effects of acute treatment with (i) AZD8418 on nicotine self-administration (n = 12) and food self-administration (n = 10) and (ii) AZD8529 on nicotine self-administration (n = 12) and food self-administration (n = 7). At least 5 days elapsed between drug administrations. Experiment 2: effects of chronic AZD8418 and AZD8529 treatment on nicotine and food self-administration [1] The effects of 14-day repeated AZD8418 and AZD8529 treatment regimens on nicotine and food self-administration were assessed using a between-subjects design. Four groups of naive rats were used to examine the effects of chronic treatment with (i) AZD8418 (0, 3.73, 7.46, and 14.92 mg/kg/day) on nicotine self-administration (n = 10–11/subgroup), (ii) AZD8418 (0 and 14.92 mg/kg/day) on food-self-administration (n = 10–11/subgroup), (iii) AZD8529 (0 and 58.3 mg/kg/day) on nicotine self-administration (n = 10–12/subgroup), and (iv) AZD8529 (0 and 58.3 mg/kg/day) on food self-administration (n = 8–13/subgroup). The subgroups for each tested compound were balanced for weight and nicotine/food intake before initiating the chronic treatments. Experiment 3: effects of acute AZD8418 and AZD8529 treatment on cue-induced reinstatement of nicotine- and food-seeking behavior [1] After completing experiment 1, nicotine self-administering rats were tested under extinction conditions. All of the rats reached the predetermined criterion of extinction (see above) by the end of the 10th extinction session. The first reinstatement session was conducted after vehicle administration to ensure that each subject exhibited robust reinstatement as defined above (>50 % increase in responding compared to the mean of the last three extinction sessions) before initiating the drug treatments. Only rats that exhibited robust nicotine-seeking behavior during this first reinstatement session were included in the remainder of the experiments. Each reinstatement session was preceded by three daily extinction sessions to re-extinguish responding. AZD8418 (0, 1.12, 3.73, 7.46, and 14.92 mg/kg) and AZD8529 (0, 1.75, 5.83, 17.5, and 58.3 mg/kg) were administered prior to each reinstatement session using a within-subjects Latin square design. Independent naive rats (n = 9–13/group) that were trained to self-administer food were used to assess the effects of AZD8418 (0, 3.73, 7.46, and 14.92 mg/kg) and AZD8529 (0, 1.75, 5.83, 17.5, and 58.3 mg/kg) on cue-induced reinstatement of food-seeking behavior using a between-subjects design because food-seeking behavior exhibits rapid extinction with repeated reinstatement testing (Bespalov et al. 2005). The groups were balanced for weight and food responding before treatment. Pharmacokinetic studies [1] AZD8529 (4.7 mg/kg) and AZD8418 (5 mg/kg) were administered orally by gavage to groups of male Wistar rats (n = 3–4), either as a single dose or as daily doses for 7 days. Tail vein blood samples (0.25 ml) were collected from all rats at 0.5, 1, 3, 6, 12, and 24 h after drug administration on day 1 or 7 of dosing. The plasma was prepared by centrifugation at 4 °C for 10 min at 1500×g within 30 min of blood sampling and analyzed for concentrations of AZD8529 or AZD8418 by a standard reverse-phase liquid chromatography and electrospray ionization tandem mass spectrometry (LC/MS/MS) method. AZD8418 was dissolved in 20 % (2-hydroxypropyl)-β-cyclodextrin and administered by oral gavage (p.o.) 1 h prior to testing. |
| 药代性质 (ADME/PK) |
PK profiles of AZD8418 and AZD8529 [1]
Peak plasma concentrations (T max) of AZD8418 were reached at 1 h post-administration after a single dose of 5 mg/kg. Peak plasma concentrations of AZD8529 were reached at 3 h post-administration after a single dose of 4.7 mg/kg. The peak plasma concentration (C max) for AZD8418 (690 ± 108 nM) was considerably higher than that for AZD8529 (158 ± 30 nM). Similarly, the area under the curve (AUC) for AZD8418 was also higher than that for AZD8529, indicating higher bioavailability of AZD8418 than that of AZD8529. Repeated daily administration of AZD8418 or AZD8529 for 7 days did not alter the T max or C max of plasma exposure. Based on findings of the PK studies, doses and pretreatment time were determined for AZD8418 (0, 0.37, 1.12, 3.73, 7.46, and 14.92 mg/kg; 1 h pretreatment) and AZD8529 (0, 1.75, 5.83, 17.5, and 58.3 mg/kg; 3 h pretreatment) administration to reflect differences in C max and T max. |
| 参考文献 | |
| 其他信息 |
Azd8418 is under investigation in clinical trial NCT01027234 (This Study Will Assess the Safety and Tolerability of AZD8418 After Single Increasing Oral Doses).
AZD-8418 is a small molecule drug with a maximum clinical trial phase of I.
Rationale: Numerous medication development strategies seek to decrease nicotine consumption and prevent relapse to tobacco smoking by blocking glutamate transmission. Decreasing glutamate release by activating presynaptic inhibitory metabotropic glutamate (mGlu)2/3 receptors inhibits the reinforcing effects of nicotine and blocks cue-induced reinstatement of nicotine-seeking behavior in rats. However, the relative contribution of mGlu2 receptors in nicotine dependence is still unknown. Objectives: The present study evaluated the role of mGlu2 receptors in nicotine-taking and nicotine-seeking behavior using the novel, relatively selective mGlu2 positive allosteric modulators (PAMs) AZD8418 and AZD8529. [1] Acute administration of the novel selective mGlu2 receptor PAMs AZD8418 and AZD8529 decreased nicotine but not food self-administration. Chronic administration of these two compounds decreased nicotine taking and food taking, although the patterns of results were different for the two compounds. Specifically, chronic treatment with AZD8418 attenuated nicotine and food self-administration but tolerance to its effect on nicotine developed quickly. Chronic AZD8529 administration resulted in a larger effect on nicotine self-administration, compared to the effect on food self-administration, and this large effect persisted throughout the 14 days of drug treatment without tolerance. Furthermore, acute AZD8418 and AZD8529 treatment attenuated cue-induced reinstatement of nicotine- and food-seeking behavior. Similarly, subchronic administration of AZD8529 also decreased cue-induced reinstatement of nicotine and food seeking although the effects were more pronounced for the reinstatement of nicotine seeking than for the reinstatement of food seeking. Altogether, these results indicate that mGlu2 receptors play an important role in the reinforcing properties of nicotine and cue-induced reinstatement of nicotine seeking. [1] |
| 精确质量 |
373.12
|
|---|---|
| 元素分析 |
C, 61.05; H, 5.39; Cl, 9.48; N, 11.24; O, 12.84
|
| CAS号 |
1198309-73-7
|
| 相关CAS号 |
1198309-73-7;
|
| PubChem CID |
44556193
|
| 外观&性状 |
Solid powder
|
| LogP |
2.7
|
| tPSA |
66.6Ų
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
582
|
| 定义原子立体中心数目 |
1
|
| InChi Key |
MCPBSUCAISQZQK-JTQLQIEISA-N
|
| InChi Code |
InChI=1S/C19H20ClN3O3/c1-10(11-4-5-11)23-9-13-6-12(7-14(20)17(13)19(23)25)16-8-15(21-26-16)18(24)22(2)3/h6-8,10-11H,4-5,9H2,1-3H3/t10-/m0/s1
|
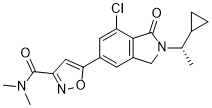
| 化学名 |
5-[7-chloro-2-[(1S)-1-cyclopropylethyl]-1-oxo-3H-isoindol-5-yl]-N,N-dimethyl-1,2-oxazole-3-carboxamide
|
| 别名 |
AZD-8418, AZD8418; AZD-8418; AZD8418; 1198309-73-7; UNII-7U6B825568; 7U6B825568; Azd 8418; 5-(7-Chloro-2-((S)-1-cyclopropyl-ethyl)-1-oxo-2,3-dihydro-1H-isoindol-5-yl)-isoxazole-3-carboxylic acid dimethylamide; AZD 8418 [WHO-DD]; AZD 8418;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。