| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
| 靶点 |
JNK (Ki = 25-50 nM)
Human JNK1 (IC50 = 10 nM, determined by kinase activity assay) [1] - Human JNK2 (IC50 = 15 nM, determined by kinase activity assay) [1] - Human JNK3 (IC50 = 8 nM, determined by kinase activity assay) [1] |
|---|---|
| 体外研究 (In Vitro) |
将 CC-401 与其他密切相关的激酶进行比较,例如 p38、细胞外信号调节激酶 (ERK)、B 激酶抑制剂 (IKK2)、蛋白激酶 C、Lck 和 70 kDa zeta 相关蛋白 (ZAP70)、JNK表现出至少 40 倍的选择性。在基于细胞的检测中,1 至 5 μM 的 CC-401 特异性抑制 JNK。 CC-401 是一种小分子,是所有三种 JNK 亚型的特异性抑制剂。当药物 CC-401 以竞争性方式结合 JNK 中的 ATP 结合位点时,转录因子 c-Jun 的 N 端激活结构域被阻止磷酸化。利用 HK-2 人肾小管上皮细胞系的渗透压,在体外检查了该抑制剂的特异性。 CC-401 以剂量依赖性方式阻止山梨醇诱导 c-Jun 磷酸化。然而,CC-401 不会抑制山梨醇引起的 JNK、p38 或 ERK 磷酸化[1]。
浓度依赖性抑制JNK1、JNK2、JNK3激酶活性,浓度高达1 μM时对其他MAP激酶(ERK1/2、p38)无显著抑制作用[1] - 1 μM CC-401使TGF-β1诱导的肾小管上皮细胞(NRK-52E)凋亡减少约60%,伴随c-Jun磷酸化水平降低约75%,Bax/Bcl-2比值下调约50%[1] - 1 μM浓度下,抑制TGF-β1诱导的NRK-52E细胞中纤连蛋白和α-SMA表达,分别减少约45%和55%,抑制肾纤维化相关蛋白合成[1] - 增强缺氧结肠癌细胞(HCT116、SW480)对DNA损伤剂(5-FU、奥沙利铂)的敏感性:0.5 μM CC-401通过阻断JNK介导的DNA修复,使5-FU诱导的细胞死亡增加约60%,奥沙利铂诱导的凋亡增加约50%[2] - 1 μM浓度下抑制结肠癌细胞中缺氧诱导的JNK磷酸化(p-JNK)约80%,阻断缺氧肿瘤细胞的存活信号通路[2] - 阻止抗GBM抗体诱导的肾小球上皮细胞(GEC)凋亡:1 μM CC-401使caspase-3激活减少约65%,GEC活力提高约55%[3] |
| 体内研究 (In Vivo) |
与对照相比,贝伐单抗、奥沙利铂和 CC-401 治疗均略微增加了 p-JNK 的染色,并且 CC-401 处理的样品中 p-cJun 含量显着降低,表明 JNK 抑制有效。与 CC-401 联合治疗会略微增加 DNA 损伤[2]。与第 14 天和第 21 天的载体组和未治疗组相比,CC-401 治疗第 7 天至第 24 天期间蛋白尿进展得更慢。然而,CC-401 治疗组大鼠在第 21 天的蛋白尿仍然更严重就严重性而言,比第 5 天时要严重。血清肌酐升高证明,载体组和未治疗组在第 24 天出现肾损伤。CC-401 治疗可以防止这种情况发生[3]。
在小鼠单侧输尿管结扎(UUO)诱导的肾纤维化模型中,口服给予CC-401(30 mg/kg,每日一次,持续14天),与溶媒对照组相比,肾间质纤维化面积减少约50%,肾小管细胞凋亡(TUNEL阳性细胞)减少约60%[1] - 改善UUO小鼠肾功能:血清肌酐和尿素氮水平分别降低约35%和30%,肾组织中纤连蛋白和α-SMA表达下调(分别约45%和50%)[1] - 在大鼠抗GBM肾小球肾炎模型中,诱导后第7天开始腹腔注射CC-401(10 mg/kg,每日一次,持续14天),阻止疾病进展:肾小球新月体形成减少约70%,蛋白尿减少约65%[3] - 保护抗GBM大鼠肾实质:减少肾小球硬化和间质炎症,存活的肾小球上皮细胞数量增加约55%[3] - 在裸鼠HCT116结肠癌异种移植模型中,口服CC-401(30 mg/kg,每日一次)联合5-FU(10 mg/kg,腹腔注射,每周两次),肿瘤生长抑制率约75%,而5-FU单药组抑制率约30%[2] |
| 酶活实验 |
JNK激酶活性测定:将重组人JNK1/JNK2/JNK3蛋白与ATP、荧光标记的c-Jun肽底物及不同浓度的CC-401在激酶反应缓冲液中孵育。30°C反应45分钟后,加入激酶终止液终止反应。荧光酶标仪检测c-Jun底物的磷酸化程度,以溶媒对照组为基准计算抑制率并确定IC50值[1]
- MAP激酶选择性测定:重组ERK1/2、p38α及JNK亚型与各自的肽底物和CC-401(0.1-1000 nM)孵育。采用相同的荧光法检测激酶活性,通过比较不同激酶的IC50值确定选择性[1] |
| 细胞实验 |
在补充有 10% FCS、10 ng/mL EGF 和 10 μg/mL 牛垂体提取物的 DMEM/F12 培养基中培养人 HK-2 近端肾小管上皮细胞。将细胞接种到六孔板中并使其粘附过夜。第二天,将培养基更换为仅补充 0.5% FCS 的 DMEM/F12,此时细胞已汇合。用在柠檬酸(pH 5.5)中制备的 CC-401 处理汇合细胞,柠檬酸在添加 300 mM 山梨糖醇前 1 小时添加。 30 分钟后使用尿素-RIPA 缓冲液收获细胞。进行了三个实验,每个实验针对每种条件进行了两次重复。对于 ELISA 测试,将 HK-2 细胞接种到 24 孔板中,给予贴壁过夜的时间,在含有 0.5% FCS 的 DMEM/F12 中培养 24 小时,然后暴露于 CC-401 或载体 60 分钟,然后进行用 1 μM 血管紧张素 II (AngII) 刺激。 48小时后,收集上清液,并使用商业ELISA试剂盒来测量TGF-β1的存在。三个实验中每个实验均使用六次重复[1]。
肾小管上皮细胞(NRK-52E)凋亡与纤维化实验:NRK-52E细胞接种于6孔板,用CC-401(0.1-10 μM)预处理1小时后,加入TGF-β1(5 ng/mL)刺激。48小时后收集细胞,western blot分析p-c-Jun、纤连蛋白、α-SMA、Bax和Bcl-2的表达;Annexin V-FITC/PI染色后流式细胞术检测凋亡细胞[1] - 缺氧结肠癌细胞敏感性实验:HCT116/SW480细胞在缺氧培养箱(1% O2)中培养24小时,随后用CC-401(0.1-5 μM)和5-FU/奥沙利铂(单药IC50浓度)处理72小时。MTT法检测细胞活力,TUNEL染色定量凋亡[2] - 肾小球上皮细胞(GEC)保护实验:分离大鼠GEC接种于24孔板,用CC-401(0.1-10 μM)预处理1小时后,加入抗GBM抗体(10 μg/mL)孵育24小时。MTT法检测细胞活力,比色法试剂盒测定caspase-3活性[3] |
| 动物实验 |
Mice: Female adult severe combined immunodeficient mice (C.B.17 SCID), which are 8–10 weeks old, are used to evaluate the effectiveness of CC-401 in inhibiting JNK signaling in anti-angiogenic and Oxaliplatin combination therapy in a mouse xenograft model. HT29 cells (1×106 cells) are subcutaneously injected into the left flank of the mice to produce tumors. To treat the mice with bevacizumab, oxaliplatin, CC401, and the proper combinations of bevacizumab, oxaliplatin, and CC-401, the tumors were divided into eight groups of eight mice each when they reached a size of about 200 mm3. The intraperitoneal injection of 5 mg/kg of bevacizumab is given to mice in the bevacizumab treatment group every three days for 21 days. The Oxaliplatin treatment group receives weekly intraperitoneal injections of 5 mg/kg Oxaliplatin for two weeks. The CC-401 treatment group is injected intraperitoneally 25 mg/kg for every 3 days. The combination treatment groups are given Bevacizumab (5 mg/kg every 3 days), Oxaliplatin (5 mg/kg every week for 2 weeks), and CC-401 (25 mg/kg every 3 days). In the control group, intraperitoneal saline is administered. Every three days, the body's weight and tumor volume are measured. The tumor volume is determined. The time difference between control and treated tumors to grow from 200 to 800 mm3 is used to calculate the tumor growth delay. In order to calculate the tumor growth delay, mice were given treatments until the tumor volume reached 800 mm3. Mice are sacrificed for immunohistochemistry on day 9 after treatments for tumor processing and staining.
Rats: Female WKY rats weighing 180–220 g are employed. Injections of sheep anti-rat GBM serum are administered intravenously five days later (referred to as day 0), after groups of nine or ten rats have received subcutaneous injections of 5 mg of sheep IgG in Freund's complete adjuvant. In this study, treatment with CC-401 (200 mg/kg/b.i.d. by oral gavage) or the control (sodium citrate) is started seven days after anti-GBM serum administration and continued twice daily until the animals are killed on day 24. At day 7 or day 24 following injection of the anti-GBM serum, additional groups of untreated rats are put to death as a control. On days 5, 14, and 21, urine is collected from the animals while they are kept in metabolic cages for 22 hours. When someone dies, blood is drawn. Urinary protein and serum creatinine are both analyzed. Mouse UUO-induced renal fibrosis model: 6-8 week-old C57BL/6 mice were subjected to unilateral ureteral ligation. CC-401 was suspended in 0.5% carboxymethylcellulose sodium and administered orally at 30 mg/kg, once daily for 14 days, starting immediately after surgery. Mice were euthanized on day 15, and kidney tissues were collected for histological analysis (HE, Masson staining), TUNEL assay, and western blot [1] - Rat anti-GBM glomerulonephritis model: 8-10 week-old Wistar rats were immunized with rabbit anti-rat GBM antibody to induce glomerulonephritis. CC-401 was dissolved in normal saline and administered intraperitoneally at 10 mg/kg, once daily for 14 days, starting at day 7 post-immunization. Urine samples were collected weekly for proteinuria measurement. Rats were euthanized on day 21, and kidney tissues were processed for histological and immunohistochemical analysis [3] - Nude mouse colon cancer xenograft model: 6-8 week-old BALB/c nude mice were subcutaneously injected with 1×106 HCT116 cells. When tumors reached ~100 mm3, mice were randomly divided into four groups: vehicle, CC-401 alone (30 mg/kg oral, daily), 5-FU alone (10 mg/kg intraperitoneal, twice weekly), and combination group. Treatment lasted for 21 days. Tumor volume was measured every 3 days, and tumors were excised for weight measurement and western blot analysis of p-JNK [2] |
| 药代性质 (ADME/PK) |
Oral bioavailability in mice was ~40% after a single 30 mg/kg dose; peak plasma concentration (Cmax) = 2.1 μg/mL at 1 hour post-administration [1]
- Plasma half-life (t1/2) in mice = 2.5 hours; distributed to kidney and tumor tissues, with tissue/plasma concentration ratios of ~1.8 (kidney) and ~1.5 (tumor) at 2 hours post-administration [1, 2] - Metabolized in the liver via cytochrome P450-mediated oxidation; ~70% of the dose was excreted in urine as metabolites within 24 hours [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In vitro cytotoxicity: No significant toxicity to normal renal tubular cells (NRK-52E) or human fibroblasts at concentrations ≤10 μM [1]
- Acute toxicity: LD50 = 350 mg/kg (oral in mice); LD50 = 180 mg/kg (intraperitoneal in mice) [1] - Subchronic toxicity: Daily oral administration of 30 mg/kg for 28 days in mice did not cause significant changes in body weight, hematological parameters, or liver/kidney function; no histological abnormalities were observed in major organs [1, 2] - Plasma protein binding rate = ~90% in humans [1] |
| 参考文献 |
|
| 其他信息 |
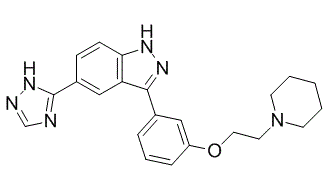
3-[3-[2-(1-piperidinyl)ethoxy]phenyl]-5-(1H-1,2,4-triazol-5-yl)-1H-indazole is a member of pyrazoles and a ring assembly.
CC-401 has been used in trials studying the treatment of Myeloid Leukemia. JNK Inhibitor CC-401 is a second generation ATP-competitive anthrapyrazolone c-Jun N terminal kinase (JNK) inhibitor with potential antineoplastic activity. Based on the chemistry of SP600125, another anthrapyrazolone inhibitor of JNK, CC-401 competitively binds the ATP binding site of JNK, resulting in inhibition of the phosphorylation of the N-terminal activation domain of transcription factor c-Jun; decreased transcription activity of c-Jun; and a variety of cellular effects including decreased cellular proliferation. CC-401 is a potent and selective small-molecule inhibitor of c-Jun amino-terminal kinase (JNK), targeting JNK1, JNK2, and JNK3 isoforms [1, 2, 3] - Its mechanism of action involves binding to the ATP-binding pocket of JNK, inhibiting JNK phosphorylation and subsequent activation of downstream substrates (e.g., c-Jun), thereby blocking JNK-mediated apoptosis, fibrosis, and tumor cell survival signaling [1, 2, 3] - Potential therapeutic applications include renal fibrosis, anti-GBM glomerulonephritis, and solid tumors (in combination with DNA-damaging chemotherapeutic agents) [1, 2, 3] - Shows favorable in vivo efficacy and low toxicity, making it a promising candidate for clinical development in JNK-mediated diseases [1, 3] |
| 分子式 |
C22H24N6O
|
|
|---|---|---|
| 分子量 |
388.47
|
|
| 精确质量 |
388.201
|
|
| 元素分析 |
C, 68.02; H, 6.23; N, 21.63; O, 4.12
|
|
| CAS号 |
395104-30-0
|
|
| 相关CAS号 |
CC-401 hydrochloride;1438391-30-0
|
|
| PubChem CID |
10430360
|
|
| 外观&性状 |
Solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
681.5±65.0 °C at 760 mmHg
|
|
| 闪点 |
366.0±34.3 °C
|
|
| 蒸汽压 |
0.0±2.1 mmHg at 25°C
|
|
| 折射率 |
1.660
|
|
| LogP |
3.74
|
|
| tPSA |
82.72
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
516
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
C1(C2=CC=CC(OCCN3CCCCC3)=C2)=NNC4=C1C=C(C=C4)C5=NC=NN5
|
|
| InChi Key |
XDJCLCLBSGGNKS-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C22H24N6O/c1-2-9-28(10-3-1)11-12-29-18-6-4-5-16(13-18)21-19-14-17(22-23-15-24-27-22)7-8-20(19)25-26-21/h4-8,13-15H,1-3,9-12H2,(H,25,26)(H,23,24,27)
|
|
| 化学名 |
3-[3-(2-piperidin-1-ylethoxy)phenyl]-5-(1H-1,2,4-triazol-5-yl)-1H-indazole
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5742 mL | 12.8710 mL | 25.7420 mL | |
| 5 mM | 0.5148 mL | 2.5742 mL | 5.1484 mL | |
| 10 mM | 0.2574 mL | 1.2871 mL | 2.5742 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00126893 | Terminated | Drug: CC-401 | Myeloid Leukemia | Celgene Corporation | October 2005 | Phase 1 |
|
|
|