| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
剂量范围为 0.09 至 12.5 微克/毫升的头孢匹林可抑制金黄色葡萄球菌 [2]。头孢匹林,也称为头孢匹林,对表皮葡萄球菌、草绿色链球菌、化脓性链球菌和肺炎双球菌的分离株的抑制作用小于 1 μg/ml [2]。
|
|---|---|
| 体内研究 (In Vivo) |
头孢匹林(200 毫克;肌肉注射;奶牛)可用于治疗奶牛的金黄色葡萄球菌感染 [3]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
... PENETRATION OF CEPHALOSPORINS INTO /CEREBROSPINAL FLUID/ IS POOR. /CEPHALOSPORINS/ CLOSE TO 50% OF CEPHAPIRIN IS BOUND TO PLASMA PROTEIN. HALF-LIFE ... IN NORMAL INDIVIDUALS IS ABOUT 40 MIN & IS DEPENDENT ON RENAL FUNCTION. SIGNIFICANT AMT ... PRESENT IN BLOOD IS REMOVED BY HEMODIALYSIS. ... EXCRETED MAINLY BY KIDNEY; ONLY 1% PRESENT IN BILE. ... /CEPHAPIRIN/ NOT ABSORBED FROM GI TRACT. IM INJECTION OF 0.5 G OF CEPHAPIRIN PRODUCES MAX PLASMA CONCN OF ABOUT 10 UG/ML @ 45 MIN; 1 G ABOUT 16 UG/ML. PLASMA CONCN ... EFFECTIVE AGAINST MANY SENSITIVE MICROORGANISMS ARE STILL DETECTABLE 6 HR AFTER SINGLE IM DOSE OF 1 G. ABOUT 30% OF IM DOSE OF CEPHAPIRIN IS EXCRETED IN URINE IN EACH OF 1ST 2 HR PERIODS AFTER INJECTION. For more Absorption, Distribution and Excretion (Complete) data for CEPHAPIRIN (8 total), please visit the HSDB record page. Metabolism / Metabolites Major metabolite detected is desacetylcephapirin. MAJOR METABOLITE OF CEPHAPIRIN IS DEACETYLCEPHAPIRIN, WHICH IS ABOUT ONE HALF OF ANTIMICROBIAL ACTIVITY OF PARENT CMPD; 20% OF ANTIBIOTIC ACTIVITY IN PLASMA IS DUE TO DEACETYLATED CMPD. Cephapirin is partially metabolized in the plasma, liver and kidneys to diacetyl cephapirin, which has about 50% of the antibacterial activity of the parent cmpd. ... Up to 20% of the antibiotic activity in serum is due to the desacetyl metabolite. Biological Half-Life EIGHT HEALTHY ADULTS RECEIVED 1 G, IV AND IM. BIOLOGICAL HALF-LIFE OF CEPHAPIRIN WAS 43 MIN. ABSORPTION HALF-LIFE OF CEPHAPIRIN FROM IM ADMIN WAS 1.25 HR. IV SODIUM CEPHAPIRIN SHOWED BIEXPONENTIAL DISPOSITION CHARACTERISTICS IN SERUM & SUBCHONDRAL BONE, REACHING DISTRIBUTION EQUIL WITHIN 20 MIN. RAPIDLY ELIMINATED, WITH BETA HALF-LIFE OF ABOUT 0.3 HR & BODY CLEARANCE OF 400 ML/MIN. Serum half-life is 0.6 hr /From table/ |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
PROBENECID & SULFINPYRAZONE COMPETE WITH URIC ACID @ RENAL TUBULE TRANSPORT SITES. URINARY EXCRETION OF OTHER WEAK ACIDS ALSO CAN BE AFFECTED BY THESE URICOSURIC AGENTS. ... CEPHALOSPORINS ... MAY BE AFFECTED BY CONCURRENT USE OF PROBENECID OR SULFINPYRAZONE. /CEPHALOSPORINS/ The admixture of beta-lactam antibacterials (penicillins and cephalosporins) and aminoglycosides may result in substantial mutual inactivation. If they are administered concurrently, they should be administered in separate sites. Do not mix them in the same intravenous bag or bottle. /Cephalosporins/ |
| 参考文献 |
[1]. Bran JL, et al. Clinical and in vitro evaluation of cephapirin, a new cephalosporin antibiotic. Antimicrob Agents Chemother. 1972 Jan;1(1):35-40.
[2]. Axelrod J, et, al. Cephapirin: in vitro antibacterial spectrum. Appl Microbiol. 1971 Nov;22(5):904-8. [3]. Roy JP, et, al. Efficacy of a 5-day extended therapy program during lactation with cephapirin sodium in dairy cows chronically infected with Staphylococcus aureus. Can Vet J. 2009 Dec;50(12):1257-62. |
| 其他信息 |
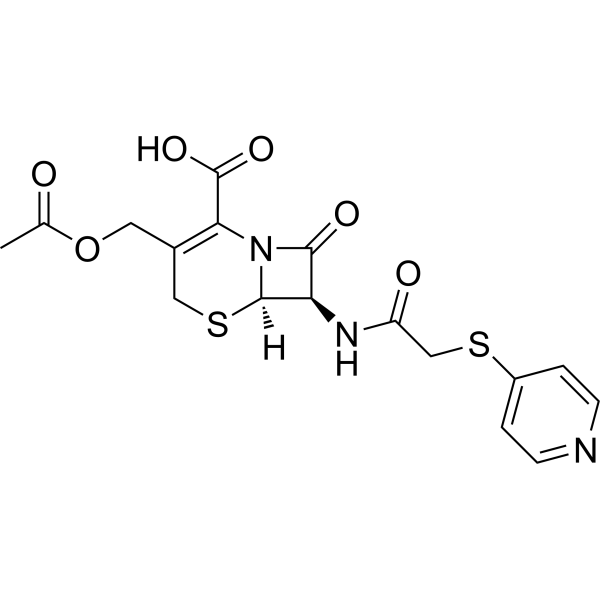
Cephapirin is a cephalosporin with acetoxymethyl and 2(pyridin-4-ylsulfanyl)acetamido substituents at positions 3 and 7, respectively, of the cephem skeleton. It is used (as its sodium salt) as an antibiotic, being effective against gram-negative and gram-positive organisms. It has a role as an antibacterial drug. It is a conjugate acid of a cephapirin(1-).
Cefapirin (INN, also spelled cephapirin), commonly marketed under the trade name Cefadyl, is a first-generation cephalosporin antibiotic that is available in injectable formulations. Production for use in humans has been discontinued in the United States. Cefapirin is partly plasma-bound and is effective against gram-negative and gram-positive organisms. Cephapirin has been reported in Apis cerana with data available. Cephapirin is a semisynthetic, broad-spectrum, first-generation cephalosporin with antibacterial activity. Cephapirin binds to and inactivates penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall. PBPs are enzymes involved in the terminal stages of assembling the bacterial cell wall and in reshaping the cell wall during growth and division. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This results in the weakening of the bacterial cell wall and causes cell lysis. Cephalosporin antibiotic, partly plasma-bound, that is effective against gram-negative and gram-positive organisms. See also: Cephapirin Sodium (has salt form); Cephapirin Benzathine (active moiety of). Drug Indication For treatment of infections caused by susceptible bacteria. Mechanism of Action The bactericidal activity of cephapirin results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins (PBPs). ... VERY RESISTANT TO ACTION OF PENICILLINASE, FOR WHICH IT IS BOTH COMPETITIVE & NONCOMPETITIVE INHIBITOR ... IT DOES NOT SUPPRESS BREAKDOWN OF PENICILLIN G BY STAPHYLOCOCCAL PENICILLINASE. CEPHALOSPORIN C & ITS SEMISYNTHETIC CONGENERS INDUCE SYNTH OF PENICILLINASE BY B CEREUS & STAPH AUREUS. /CEPHALOSPORIN C/ ... ENZYME ACTING SPECIFICALLY ON CEPHALOSPORIN C TO DESTROY ITS ANTIBACTERIAL ACTIVITY. THIS SUBSTANCE, CEPHALOSPORINASE IS ALSO BETA-LACTAMASE. MOST PREPN OF ENZYME ALSO EXHIBIT PENICILLINASE ACTIVITY, & SOME MICROORGANISMS PRODUCE ONE BETA-LACTAMASE THAT ACTS ON BOTH PENICILLIN & CEPHALOSPORINS. /CEPHALOSPORIN C/ Bactericidal; action depends on ability to reach and bind pencillin-binding proteins located in bacterial cytoplasmic membranes; cephalosporins inhibit bacterial septum and cell wall synthesis, probably by acylation of membrane-bound transpeptidase enzymes. This prevents cross-linkage of peptidoglycan chains, which is necessary for bacterial cell wall strength and rigidity. Also, cell division and growth are inhibited, and lysis and elongation of susceptible bacteria frequently occur. Rapidly dividing bacteria are those most susceptible to the action of cephalosporins. /Cephalosporins/ Therapeutic Uses Cephalosporins Cephalosporins are still useful as alternatives to penicillins for a variety of infections in patients who can't tolerate penicillins. These include streptococcal and staphylococcal infections. BACTERIA SUSCEPTIBLE TO CEPHALOTHIN /BOTH GRAM POSITIVE & GRAM-NEGATIVE MICROORGANISMS/ ARE SENSITIVE OVER APPROX SAME RANGE OF CONCN TO ... CEPHAPIRIN. ... CEPHAPIRIN IS SOMEWHAT MORE INHIBITORY THAN CEPHALOTHIN FOR GROUP-A STREP PYOGENES & PNEUMOCOCCUS. CEPHAPIRIN IS ONE OF NEWEST SEMISYNTHETIC CEPHALOSPORINS. ... LIKE OTHER CEPHALOSPORINS, CEPHAPIRIN SHOULD BE RESERVED FOR THOSE INSTANCES IN WHICH ORGANISM IS SENSITIVE TO IT & PATIENT IS HYPERSENSITIVE TO PENICILLIN. For more Therapeutic Uses (Complete) data for CEPHAPIRIN (10 total), please visit the HSDB record page. Drug Warnings SINCE ... CEPHAPIRIN ... ADMIN AS ... SODIUM SALTS, CARE SHOULD BE EXERCISED IN USE OF LARGE DOSES IN PERSONS WITH IMPAIRED CAPACITY TO EXCRETE THIS CATION. ... SUPRAINFECTIONS, USUALLY DUE TO GRAM NEGATIVE BACTERIA, MAY OCCUR WHEN THESE ANTIBIOTICS ARE EMPLOYED. TYPICAL DOSAGE SCHEDULES ... FOR MOST OF CEPHALOSPORINS MUST BE MODIFIED FOR PATIENTS WITH IMPAIRED RENAL FUNCTION. /CEPHALOSPORINS/ ... HYPERSENSITIVITY REACTIONS TO CEPHALOSPORINS IS HIGHER IN PATIENTS WHO HAVE SHOWN ALLERGIC MANIFESTATIONS FOLLOWING ADMIN OF PENICILLIN. THIS APPEARS TO BE RELATED TO SENSITIZATION TO BETA-LACTAM RING COMMON TO BOTH THESE DRUGS. /CEPHALOSPORINS/ ... ENTEROCOCCAL ENDOCARDITIS CANNOT BE CURED WITH CEPHALOSPORIN EVEN WHEN ... GIVEN CONCURRENTLY WITH GENTAMICIN OR STREPTOMYCIN. ... ENTEROBACTER (AEROBACTER) INFECTIONS ARE, AS A RULE, RESISTANT TO THESE CMPD. /CEPHALOSPORINS/ For more Drug Warnings (Complete) data for CEPHAPIRIN (10 total), please visit the HSDB record page. Pharmacodynamics Cephapirin is a first-generation cephalosporin that has a wide spectrum of activity against gram-positive and gram-negative organisms. Cephapirin is more resistant to beta-lactamases than are the penicillins and so is effective against staphylococci, with the exception of methicillin-resistant staphylococci. |
| 分子式 |
C17H17N3O6S2
|
|---|---|
| 分子量 |
423.458
|
| 精确质量 |
423.056
|
| CAS号 |
21593-23-7
|
| 相关CAS号 |
Cephapirin sodium;24356-60-3
|
| PubChem CID |
30699
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 蒸汽压 |
6.98E-26mmHg at 25°C
|
| LogP |
1.253
|
| tPSA |
179.99
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
707
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CC(OCC1=C(N2C([C@@H](NC(CSC3=CC=NC=C3)=O)[C@H]2SC1)=O)C(O)=O)=O
|
| InChi Key |
UQLLWWBDSUHNEB-CZUORRHYSA-N
|
| InChi Code |
InChI=1S/C17H17N3O6S2/c1-9(21)26-6-10-7-28-16-13(15(23)20(16)14(10)17(24)25)19-12(22)8-27-11-2-4-18-5-3-11/h2-5,13,16H,6-8H2,1H3,(H,19,22)(H,24,25)/t13-,16-/m1/s1
|
| 化学名 |
(6R,7R)-3-(acetyloxymethyl)-8-oxo-7-[(2-pyridin-4-ylsulfanylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
|
| 别名 |
Cefapirine; Cephapirin; Cefapirin
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3615 mL | 11.8075 mL | 23.6150 mL | |
| 5 mM | 0.4723 mL | 2.3615 mL | 4.7230 mL | |
| 10 mM | 0.2361 mL | 1.1807 mL | 2.3615 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。