| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 体外研究 (In Vitro) |
1000 ng/ml 的 ceterix diacetate 可抑制 ES-2 细胞系的发育。二醋酸西特普利克的抗增殖作用与GnRH-I激动剂相似,表明癌细胞中的GnRH-I系统可能不受GnRH-I激动剂和拮抗剂二分法的影响[2]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapidly absorbed following subcutaneous injection. The mean absolute bioavailability following subcutaneous administration to healthy female subjects is 85%. Following subcutaneous administration of 10 mg cetrorelix to males and females, only unchanged cetrorelix was detected in urine. 1.16 L/kg 1.28 ml/min·kg [adult healthy female with 3 mg single SC administration] Following subcutaneous administration of 10 mg cetrorelix to males and females, only unchanged cetrorelix was detected in urine. In 24 hours, cetrorelix and small amounts of the (1-9), (1-7), (1-6), and (1-4) peptides were found in bile samples. 2-4% of the dose was eliminated in the urine as unchanged cetrorelix, while 5-10% was eliminated as cetrorelix and the four metabolites in bile. Therefore, only 7-14% of the total dose was recovered as unchanged cetrorelix and metabolites in urine and bile up to 24 hours. The remaining portion of the dose may not have been recovered since bile and urine were not collected for a longer period of time. The volume of distribution of Cetrotide following a single intravenous dose of 3 mg is about 1 L/kg. In vitro protein binding to human plasma is 86%. Cetrotide concentrations in follicular fluid and plasma were similar on the day of oocyte pick-up in patients undergoing controlled ovarian stimulation. Following subcutaneous administration of Cetrotide 0.25 mg and 3 mg, plasma concentrations of cetrorelix were below or in the range of the lower limit of quantitation on the day of oocyte pick-up and embryo transfer. Cetrotide is rapidly absorbed following subcutaneous injection, maximal plasma concentrations being achieved approximately one to two hours after administration. The mean absolute bioavailability of Cetrotide following subcutaneous administration to healthy female subjects is 85%. Pharmacokinetic studies were performed predominantly in rats and dogs. Absorption from the sc injection site was rapid and complete and there were no differences in absorption with regard to sex or species. A linear relationship between dose and plasma AUC was evident. Distribution of cetrorelix was rapid. Main target organs were the kidney, liver, small intestine and organs containing the luteinising hormone releasing hormone (LHRH) receptor (pituitary gland, ovaries). Plasma protein binding amounted to 86%. Elimination from most tissues was rapid and occurred predominantly within 48 hr. ... Cetrorelix crosses the placenta only to a low extent. The distribution of cetrorelix or its metabolites into milk was not investigated. Cetrorelix is excreted unchanged into urine and after metabolism by peptidases into bile. ... Studies in healthy volunteers indicate that cetrorelix is excreted in a similar manner in humans, rats and dogs. Following subcutaneous injection, the absolute bioavailability of cetrorelix was approximately 85% in both males and females. The apparent volume of distribution was 1.16 + or - 0.29 L/kg in females and 1.02 + or - 0.33 L/kg in males. The terminal half-life was about 10 hours after iv and 30 hours after subcutaneous injection with a trend towards lower values in female. Protein binding in human plasma was around 85%. Linear pharmacokinetics were observed following both single (0.25, 0.5 and 1.00 mg) and multiple dose administration (0.25 to 1.00 mg). The pharmacokinetics were linear up to a 3 mg dose. Metabolism / Metabolites In in vitro studies, cetrorelix was stable against phase I- and phase II-metabolism. Cetrorelix was transformed by peptidases, and the (1-4) peptide was the predominant metabolite. The main metabolite of cetrorelix in the rat bile was identified as being the heptapeptide (1-7). The metabolite was pharmacologically inactive in rats, in terms of testosterone suppression. After subcutaneous administration of 10 mg Cetrotide to females and males, Cetrotide and small amounts of (1-9), (1-7), (1-6), and (1-4) peptides were found in bile samples over 24 hours. In in vitro studies, Cetrotide was stable against phase I- and phase II-metabolism. Cetrotide was transformed by peptidases, and the (1-4) peptide was the predominant metabolite. Biological Half-Life ~62.8 hours In humans, the terminal half-life values after iv and sc administration were 8-9 hr and 24-40 hr, respectively. The terminal half-lives in rats after iv and sc administration were 1-2 hr and 7-14 hr, respectively ... . Elimination half life: Single 3 mg dose: 62.8 hr (38.2-108 hr); Single 0.25 mg dose: 5.0 hr (2.4-48.8 hr); 0.25 mg daily for 14 days: dose: 20.6 hr (4.1-179.3 hr) /From table/ Half-lives of greater than or equal to 100 hr were observed mainly in organs of elimination (liver, kidney), spleen and in the organs containing LHRH binding sites. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
86% Non-Human Toxicity Values 68.1 mg/kg was determined as the minimal lethal dose. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Cetrorelix is indicated for the inhibition of premature luteinizing hormone (LH) surges in women undergoing controlled ovarian stimulation. /EXPL THER/ This randomized, placebo-controlled, single-blind, experimental study was performed on 45 Wistar adult female rats ... . After the peritoneal implantation of endometrial tissue, rats were randomized to three equal intervention groups: (i) control group, (ii) leuprolide group, and (iii) cetrorelix group. Six weeks later, following implant volume measurements (volume-1) by performing a second laparotomy, saline (0.1 mL/rat) was administered subcutaneously to the control group once a week, leuprolide (0.075 mg/kg) subcutaneously to the leuprolide group twice at 4-week intervals and cetrorelix (0.001 mg/rat/day) subcutaneously to the cetrorelix group for 8 weeks. At the end of the treatment, by performing a third laparotomy, implant volumes were remeasured (volume-2) and implants were totally excised for histopathological examination. The volume-1 and volume-2 values within the groups, and stromal and glandular tissue scores between the groups were compared. In both the leuprolide group and the cetrorelix group, volume-2 as compared to volume-1 had significantly reduced (P < 0.01, P < 0.01 respectively), while there was no significant volume change in the control group (P > 0.05). In this group, when compared with the control group, glandular and stromal tissues had significantly lessened (P < 0.01, P < 0.01 respectively). Leuprolide and cetrorelix were found to have similar efficacy in the regression of both the size and the histological structure of experimental endometriotic implants. Drug Warnings Cetrorelix should be prescribed by health care providers who are experienced in fertility treatment. Before starting treatment with cetrorelix acetate, pregnancy must be excluded. Elevations in liver function test results including ALT (SGPT), AST (SGOT), gamma-glutamyltransferase (GGT, gamma-glutamyl transpeptidase, GGTP), and alkaline phosphatase up to 3 times the upper limit of normal were reported in 1-2% of patients receiving cetrorelix during controlled ovarian stimulation. Caution is advised in patients with hypersensitivity to GnRH. Carefully monitor these patients after the first injection. A severe anaphylactic reaction associated with cough, rash, and hypotension was observed in 1 patient after 7 months of treatment with 10 mg/day cetrorelix in a study for an indication unrelated to infertility. Local site reactions (e.g. redness, erythema, bruising, itching, swelling and pruritus) were reported. Usually, they were of a transient nature, mild intensity and short duration. For more Drug Warnings (Complete) data for CETRORELIX (8 total), please visit the HSDB record page. Pharmacodynamics Cetrorelix is a synthetic decapeptide with gonadotropin-releasing hormone (GnRH) antagonistic activity. GnRH induces the production and release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the gonadotrophic cells of the anterior pituitary. Due to a positive estradiol (E2) feedback at midcycle, GnRH liberation is enhanced resulting in an LH-surge. This LH-surge induces the ovulation of the dominant follicle, resumption of oocyte meiosis and subsequently luteinization as indicated by rising progesterone levels. Cetrorelix competes with natural GnRH for binding to membrane receptors on pituitary cells and thus controls the release of LH and FSH in a dose-dependent manner. |
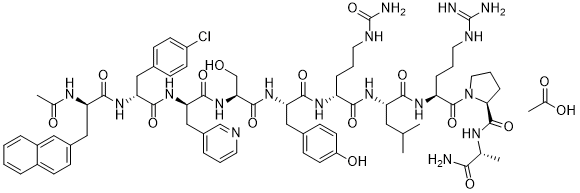
| 分子式 |
C70H92CLN17O14.2(C2H4O2)
|
|---|---|
| 精确质量 |
1429.669
|
| CAS号 |
130143-01-0
|
| 相关CAS号 |
Cetrorelix Acetate;145672-81-7;Cetrorelix;120287-85-6
|
| PubChem CID |
25074887
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
1768.4ºC at 760 mmHg
|
| 闪点 |
1023.3ºC
|
| 蒸汽压 |
0mmHg at 25°C
|
| 折射率 |
1.668
|
| LogP |
2.69
|
| tPSA |
518.21
|
| 氢键供体(HBD)数目 |
16
|
| 氢键受体(HBA)数目 |
16
|
| 可旋转键数目(RBC) |
38
|
| 重原子数目 |
102
|
| 分子复杂度/Complexity |
2840
|
| 定义原子立体中心数目 |
10
|
| SMILES |
NC(NCCCCC(N1[C@H](C(N([C@H](C)C(N(C([C@H](N)CC2=CC=C(Cl)C=C2)=O)C([C@H](CC2=CC=C(OC(C)=O)C=C2)NC([C@H](COC(C)=O)NC([C@H](N)CC2=CN=CC=C2)=O)=O)=O)=O)C([C@H](NC(C)=O)CC2=CC3=CC=CC=C3C=C2)=O)=O)CCC1)=O)=N
|
| InChi Key |
SBNPWPIBESPSIF-MHWMIDJBSA-N
|
| InChi Code |
InChI=1S/C70H92ClN17O14/c1-39(2)31-52(61(94)82-51(15-9-28-77-69(73)74)68(101)88-30-10-16-58(88)67(100)79-40(3)59(72)92)83-60(93)50(14-8-29-78-70(75)102)81-63(96)54(34-43-20-25-49(91)26-21-43)86-66(99)57(38-89)87-65(98)56(36-45-11-7-27-76-37-45)85-64(97)55(33-42-18-23-48(71)24-19-42)84-62(95)53(80-41(4)90)35-44-17-22-46-12-5-6-13-47(46)32-44/h5-7,11-13,17-27,32,37,39-40,50-58,89,91H,8-10,14-16,28-31,33-36,38H2,1-4H3,(H2,72,92)(H,79,100)(H,80,90)(H,81,96)(H,82,94)(H,83,93)(H,84,95)(H,85,97)(H,86,99)(H,87,98)(H4,73,74,77)(H3,75,78,102)/t40-,50-,51+,52+,53-,54+,55-,56-,57+,58+/m1/s1
|
| 化学名 |
(2S)-1-[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2R)-2-[[(2R)-2-acetamido-3-naphthalen-2-ylpropanoyl]amino]-3-(4-chlorophenyl)propanoyl]amino]-3-pyridin-3-ylpropanoyl]amino]-3-hydroxypropanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-5-(carbamoylamino)pentanoyl]amino]-4-methylpentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]-N-[(2R)-1-amino-1-oxopropan-2-yl]pyrrolidine-2-carboxamide
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Effect of GnRH Agonist vs GnRH Antagonist on Oocyte Morphology During IVF/ICSI
CTID: NCT04724486
Phase: Phase 4 Status: Completed
Date: 2023-10-24