| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g | |||
| 100g | |||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oral bioavailability is nearly complete, at approximately 90%, and peak serum concentrations (Cmax) of, on average, 2.50 µg/mL are reached at 0.75 hours (Tmax). The AUC following an orally administered dose of 300mg was found to be approximately 11 µg•hr/mL. Systemic exposure from the administration of vaginal suppository formulations is 40-fold to 50-fold lower than that observed following parenteral administration and the Cmax observed following administration of vaginal cream formulations was 0.1% of that observed following parenteral administration. Approximately 10% of clindamycin bioactivity is excreted in the urine and 3.6% in the feces, with the remainder excreted as inactive metabolites. Clindamycin is widely distributed in the body, including into bone, but does not distribute into cerebrospinal fluid. The volume of distribution has been variably estimated between 43-74 L. The plasma clearance of clindamycin is estimated to be 12.3-17.4 L/h, and is reduced in patients with cirrhosis and altered in those with anemia. Clindamycin is nearly completely absorbed following oral admin. Peak plasma concn of 2 to 3 ug/mL are attained within 1 hr after the ingestion of 150 mg. The presence of food in stomach does not reduce absorption. The half-life of the antibiotic is about 2.9 hr, and the modest accumulation of drug is thus expected if it is given at 6-hr intervals. Clindamycin is widely distributed in many fluids and tissues, including bone. Significant concn are not attained in cerebrospinal fluid, even when the meninges are inflamed. Concn sufficient to treat cerebral toxoplasmosis are achievable .. The drug readily crosses the placental barrier. 90% or more of clindamycin is bound to plasma proteins. Clindamycin accumulates in polymorphonuclear leukocytes, alveolar macrophages, and in abscesses. Half-life ... is lengthened only slightly in patients with markedly impaired renal function ... Clindamycin is distributed into many body tissues and fluids including saliva, ascites fluid, pleural fluid, synovial fluid, bone, and bile. However, even in the presence of inflamed meninges, only small amounts of the drug diffuse into CSF. The concentration of clindamycin in synovial fluid and bone is reported to be 60-80% of concurrent serum concentrations of the drug; the degree of penetration does not appear to be affected by joint inflammation. Clindamycin readily crosses the placenta, and cord blood concentrations of the drug have been reported to be 46% of concurrent maternal blood concentrations. Clindamycin is distributed into milk. For more Absorption, Distribution and Excretion (Complete) data for CLINDAMYCIN (24 total), please visit the HSDB record page. Metabolism / Metabolites Clindamycin undergoes hepatic metabolism mediated primarily by CYP3A4 and, to a lesser extent, CYP3A5. Two inactive metabolites have been identified - an oxidative metabolite, clindamycin sulfoxide, and an N-demethylated metabolite, N-desmethylclindamycin. Clindamycin is partially metabolized to bioactive and inactive metabolites. The major bioactive metabolites are clindamycin sulfoxide and N-demethyl-clindamycin which are excreted in urine, bile, and feces. Within 24 hours, approximately 10% of an oral dose of clindamycin is excreted in urine and 3.6% is excreted in feces as active drug and metabolites; the remainder is excreted as inactive metabolites. Only about 10% of the clindamycin admin is excreted unaltered in urine, and small quantities are found in feces ... Clindamycin is inactivated by metabolism to N-demethylclindamycin and clindamycin sulfoxide, which are excreted in the urine and bile. Clindamycin has known human metabolites that include N-desmethyclindamycin and clindamycin sulfoxide. Biological Half-Life The elimination half-life of clindamycin is about 3 hours in adults and 2.5 hours in children. Half-life is increased to approximately 4 hours in the elderly. The serum half-life of clindamycin is 2-3 hours in adults and children with normal renal function. The serum half-life is increased slightly in patients with markedly reduced renal or hepatic function. In neonates, the serum half-life depends on gestational and chronologic age and body weight. The serum half-life of clindamycin reportedly averages 8.7 and 3.6 hours in premature and full-term neonates, respectively, and about 3 hours in infants 4 weeks to 1 year of age; serum half-life was longer in infants weighing less than 3.5 kg than in heavier infants. The half-life of ... /clindamycin/ is about 2.9 hr ... Following intravaginal application of 2% clindamycin cream, the systemic half-life of the drug appears to be about 1.5-2.6 hours. Following intravaginal administration of clindamycin suppositories, the apparent elimination half-life averaged about 11 hours (range: 4-35 hours). |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Reversibility of antibiotic-induced paralysis of mouse phrenic nerve-hemidiaphragm preparation by calcium and by neostigmine. Concurrent use of kaolin- or attapulgite-containing antidiarrheals with oral clindamycin may significantly delay the absorption of oral clindamycin; concurrent use should be avoided or patients should be advised to take adsorbent antidiarrheals not less than 2 hours before or 3 to 4 hours after oral lincomycins. There is in vitro evidence of antagonism between erythromycin and clindamycin. Clindamycin has been reported to antagonize the bactericidal activity of aminoglycosides in vitro, and some clinicians recommend that these drugs not be used concomitantly. However, in vivo antagonism has not been demonstrated, and clindamycin has been administered successfully in conjunction with an aminoglycoside with no apparent decrease in activity. For more Interactions (Complete) data for CLINDAMYCIN (7 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat subcutaneous 2618 mg/kg LD50 Rat oral 2619 mg/kg /Clindamycin hydrochloride/ LD50 Mouse ip 361 mg/kg /Clindamycin hydrochloride/ LD50 Swiss Mouse ip 1145 mg/kg /Clindamycin 2-phosphate/ LD50 Swiss Mouse iv 855 mg/kg /Clindamycin 2-phosphate/ |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Anti-Bacterial Agents Clindamycin is used intravaginally as a vaginal cream or suppository or orally for the treatment of bacterial vaginosis (formerly called Haemophilus vaginitis, Gardnerella vaginitis, nonspecific vaginitis, Corynebacterium vaginitis, or anaerobic vaginosis). /Included in US product labeling/ Clindamycin phosphate is used topically alone or in conjunction with benzoyl peroxide in the treatment of inflammatory acne vulgaris. /Clindamycin phosphate; included in US product labeling/ Clindamycin is used parenterally in the treatment of bone and joint infections (including acute hematogenous osteomyelitis) caused by Staphylococcus aureus and as an adjunct to surgery in the treatment of chronic bone and joint infections caused by susceptible organisms. Clindamycin also is used orally or parenterally in the treatment of serious respiratory tract infections, skin and skin structure infections, or septicemia caused by susceptible strains of S. aureus, Streptococcus pneumoniae, or other streptococci (except Enterococcus faecalis). /Included in US product labeling/ For more Therapeutic Uses (Complete) data for CLINDAMYCIN (23 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including CLEOCIN HCL and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C.difficle. Because CLEOCIN HCL therapy has been associated with severe colitis which may end fatally, it should be reserved for serious infections where less toxic antimicrobial agents are inappropriate... It should not be used in patients with nonbacterial infections such as most upper respiratory tract infections. C.difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated. The most frequent adverse effects of topical therapy with clindamycin phosphate 1% gel, lotion, or solution are dryness of the skin and erythema. In clinical studies evaluating the clindamycin phosphate 1% topical gel, lotion, or solution, dryness was reported in 23, 18, or 19% of patients, respectively, whereas erythema was reported in 7, 14, or 16% of patients, respectively. Oiliness or oily skin was reported in 18, 10, or 1% of patients receiving the topical gel, lotion, or solution, respectively. Peeling occurred in 7 or 11% of patients receiving topical clindamycin phosphate lotion or solution, respectively. In addition, burning or pruritus were reported in 7-11% of patients receiving these topical preparations of clindamycin phosphate. /Clindamycin phosphate/ Vaginitis (including vulvovaginitis, vulvovaginal disorder, vaginal discharge, and trichomonal vaginitis) has been reported in 3.6 or 9-10.7% of nonpregnant women receiving clindamycin phosphate vaginal suppositories or cream, respectively. Vulvovaginitis has been reported in 6 or 4.4% and vulvovaginal disorder (including irritation) has been reported in 3.2 or 5.3% of nonpregnant women receiving clindamycin phosphate vaginal cream for 3 or 7 days, respectively. Vulvovaginal disorder or vaginal pain has been reported in 3.4 or 1.9%, respectively, of nonpregnant women receiving clindamycin vaginal suppositories. Trichomonas vaginalis infection reportedly occurs in 1.3% of nonpregnant women receiving clindamycin phosphate vaginal cream for 7 days. Vaginal discharge, metrorrhagia, urinary tract infection, pyelonephritis, dysuria, endometriosis, menstrual disorder, and vaginal pain each have been reported in less than 1% of patients receiving intravaginal clindamycin, and vaginal bleeding has been reported in at least one patient following use of clindamycin phosphate vaginal cream. /Clindamycin phosphate/ The most common adverse effects of therapy with clindamycin phosphate (2% clindamycin) vaginal cream or suppositories are vaginal candidiasis and vaginitis (including vulvovaginitis, vulvovaginal disorder, vaginal discharge, and trichomonal vaginitis). /Clindamycin phosphate/ For more Drug Warnings (Complete) data for CLINDAMYCIN (35 total), please visit the HSDB record page. Pharmacodynamics Clindamycin exerts its bacteriostatic effect via inhibition of microbial protein synthesis. Clindamycin has a relatively short Tmax and half-life necessitating administration every six hours to ensure adequate antibiotic concentrations. _Clostridium difficile_ associated diarrhea (CDAD) has been observed in patients using clindamycin, ranging in severity from mild diarrhea to fatal colitis and occasionally occurring over two months following cessation of antibiotic therapy. Overgrowth of _C. difficile_ resulting from antibiotic use, along with its production of A and B toxins, contributes to morbidity and mortality in these patients. Because of the associated risks, clindamycin should be reserved for serious infections for which the use of less toxic antimicrobial agents are inappropriate. Clindamycin is active against a number of gram-positive aerobic bacteria, as well as both gram-positive and gram-negative anaerobes. Resistance to clindamycin may develop, and is generally the result of base modification within the 23S ribosomal RNA. Cross-resistance between clindamycin and lincomycin is complete, and may also occur between clindamycin and macrolide antibiotics (e.g. [erythromycin]) due to similarities in their binding sites. As antimicrobial susceptibility patterns are geographically distinct, local antibiograms should be consulted to ensure adequate coverage of relevant pathogens prior to use. |
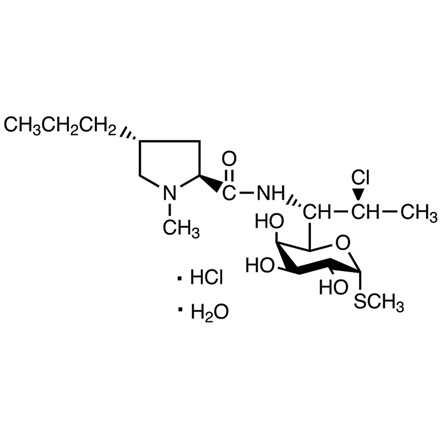
| 分子式 |
C18H34CL2N2O5S
|
|---|---|
| 分子量 |
461.4440
|
| 精确质量 |
478.167
|
| 元素分析 |
C, 45.09; H, 7.57; Cl, 14.79; N, 5.84; O, 20.02; S, 6.69
|
| CAS号 |
58207-19-5
|
| 相关CAS号 |
Clindamycin hydrochloride;21462-39-5;Clindamycin;18323-44-9
|
| PubChem CID |
446598
|
| 外观&性状 |
Yellow, amorphous solid
|
| 沸点 |
647ºC at 760 mmHg
|
| 熔点 |
143ºC
|
| 闪点 |
345.1ºC
|
| 折射率 |
143 ° (C=2, H2O)
|
| LogP |
1.456
|
| tPSA |
136.79
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
502
|
| 定义原子立体中心数目 |
9
|
| SMILES |
Cl[C@@]([H])(C([H])([H])[H])[C@]([H])([C@]1([H])[C@@]([H])([C@@]([H])([C@]([H])([C@]([H])(O1)SC([H])([H])[H])O[H])O[H])O[H])N([H])C([C@]1([H])C([H])([H])[C@@]([H])(C([H])([H])C([H])([H])C([H])([H])[H])C([H])([H])N1C([H])([H])[H])=O.Cl[H]
|
| InChi Key |
KWMXKEGEOADCEQ-WNNJHRBUSA-N
|
| InChi Code |
InChI=1S/C18H33ClN2O5S.ClH.H2O/c1-5-6-10-7-11(21(3)8-10)17(25)20-12(9(2)19)16-14(23)13(22)15(24)18(26-16)27-4;;/h9-16,18,22-24H,5-8H2,1-4H3,(H,20,25);1H;1H2/t9-,10+,11-,12+,13-,14+,15+,16+,18+;;/m0../s1
|
| 化学名 |
(2S,4R)-N-((1S,2S)-2-chloro-1-((2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(methylthio)tetrahydro-2H-pyran-2-yl)propyl)-1-methyl-4-propylpyrrolidine-2-carboxamide hydrochloride hydrate
|
| 别名 |
Clindamycin HCl (H2O); Clindamycin hydrochloride hydrate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1671 mL | 10.8356 mL | 21.6713 mL | |
| 5 mM | 0.4334 mL | 2.1671 mL | 4.3343 mL | |
| 10 mM | 0.2167 mL | 1.0836 mL | 2.1671 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Clindamycin 300 mg Capsules in Healthy Subjects Under Fed Conditions
CTID: NCT00836004
Phase: Phase 1 Status: Completed
Date: 2024-08-19