| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Thrombin
|
|---|---|
| 体外研究 (In Vitro) |
对三种商业肽的多重脱质子化离子进行了碰撞诱导解离(CID):水蛭素(54-65)/hirudin (54-65)、纤维蛋白肽B和氧化胰岛素链A。离子是通过傅里叶变换离子回旋共振质谱仪中的电喷雾电离产生的。这些肽中的每一种都含有多个酸性残基,这使得它们很难在阳性模式下电离。然而,肽很容易去质子化,使负离子研究成为一种可行的替代方案。CID光谱表明,可能的去质子化位点是酸性残基(天冬氨酸、谷氨酸和半胱氨酸)和C末端。光谱中充满了c、y和内部离子,尽管形成了一些a、b、x和z离子。许多碎片离子是由酸性残基附近的裂解形成的,包括酸性位点的N端和c端。此外,母体离子和碎片离子都普遍存在中性损失(如NH3、CH3、H2O和CO2)。这些中性消除通常指示特定的氨基酸残基。母离子的几种电荷态的碎裂模式结合在一起时,提供了重要的一级序列信息。这些结果表明,多重脱质子化离子的负模式CID提供了有用的结构信息,对于不大量形成正离子的高酸性肽来说是有价值的。[3]
在内部和外部凝血途径中,组氨酸抑制凝血酶活性,剥夺凝血酶裂解纤维蛋白原的能力,并通过交联阻止纤维蛋白形成以及纤维蛋白单体的聚合过程[1]。通过对抗凝血酶,水蛭素可减少人微血管内皮细胞的凋亡并抑制 p-JAK2 的表达 [1]。高浓度时,水蛭素可抑制人微血管内皮细胞和 VEGF-Notch 通路的生长 [1]。 TGF-β1 诱导的 HK-2 细胞异常增殖和纤维化可以通过水蛭素 (3–10 mg/mL) 来抵消 [1]。 Hirudin 抑制 ERK1/2 通路,减少氧化应激,抑制血管紧张素 II 诱导的心脏成纤维细胞,并以剂量依赖性方式调节与纤维相关的变量 [1]。 Li等人报道,当在体外靶向LN229和U251细胞系时,水蛭素/Hirudin显示出抗肿瘤作用。它显著抑制了LN229和U251细胞系的生长,IC 50分别为30和15 mM。此外,水蛭素还通过下调Bcl-2的表达和增加G1期细胞周期阻滞来增加这些胶质瘤细胞的凋亡。值得注意的是,给予水蛭素后,pERK1/2的含量降低,细胞周期蛋白D1的水平降低,这表明ERK/MAPK信号通路的下调可能是水蛭素治疗胶质瘤的关键机制(赵,2015)。 最近,发现水蛭素对肝细胞癌具有抑制作用,其潜在机制可能与抑制血管生成有关。Li等人报道,水蛭素可以以剂量依赖的方式抑制HepG2细胞的增殖、凋亡、迁移和侵袭。此外,水蛭素治疗后,VEGF mRNA和蛋白的表达显著下调(Li等人,2016),这与之前的研究一致,即肝细胞癌血管生成的减少归因于VEGF通路的失活(Morse等人,2019)。此外,水蛭素是凝血酶的特异性抑制剂,可以抑制凝血酶刺激的血管生成,降低VEGF的表达。血管瘤是由血管内皮细胞和周细胞异常增殖引起的病变,可以通过抗血管生成药物进行干预和治疗(Ji等人,2014)。2015年的一项研究表明,4U/ml水蛭素在体外能有效抑制小鼠EOMA血管瘤细胞的增殖并促进其凋亡,表现出明显的剂量-效应关系(Yang等人,2015)。 除了天然的Hirudin外,rH还通过抑制血管生成对Hep-2人喉癌症(LC)细胞具有抗肿瘤作用。[1] 水蛭素降低TGF-β诱导的肾小管上皮细胞中炎症因子的表达[2] 在人HK-2细胞、小鼠IMCD3细胞和大鼠NRK-52E细胞中诱导TGF-β后,这些细胞中IL-1β、IL-6和TNF-α的水平升高,然后当这些细胞用水蛭素从0.5mg/ml处理到1mg/ml时,这些水平逐渐降低(图5A-C)。 水蛭素可减少TGF-β诱导的肾小管上皮细胞中EMT的发生[2] E-cad、N-cad和slug是EMT发生的关键蛋白。如图6A-C所示,当HK-2细胞、IMCD3细胞和NRK-52E细胞用TGF-β处理时,N-cad和slug的表达增加,E-cad的表达减少。此外,0.5 mg/ml至1 mg/ml的水蛭素处理可以改善N-cad和slug的表达,并抑制这些细胞中E-cad的表达。 水蛭素降低TGF-β诱导的肾小管上皮细胞纤维化的发生率[2] 如图7A-C所示,TGF-β诱导的HK-2细胞、IMCD3细胞和NRK-52E细胞中胶原蛋白-I、FN和α-SMA的表达增加。当这些细胞用0.5mg/ml至1mg/ml的水蛭素处理时,细胞中胶原蛋白-I、FN和α-SMA的表达逐渐降低。 水蛭素可减少TGF-β诱导的肾小管上皮细胞的凋亡[2] 通过Hoechst染色检测HK-2细胞、IMCD3细胞和NRK-52E细胞的凋亡。TGF-β诱导的HK-2细胞、IMCD3细胞和NRK-52E细胞的凋亡增强,而当这些细胞用0.5 mg/ml至1 mg/ml的水蛭素处理时,凋亡减轻(图8A-C)。 |
| 体内研究 (In Vivo) |
在大鼠中,Hirudin可降低炎症反应并提高随机皮瓣的存活率[1]。激光手术后,Hirudin可帮助 SD 大鼠的伤口恢复 [1]。在单侧输尿管梗阻 (UUO) 小鼠模型中,Hirudin(10 和 15 mg/kg;灌胃,每天三次,持续 21 天)可减少肾间质纤维化,从而减轻肾小管损伤和炎症 [2]。
最近,越来越多的研究集中在水蛭素衍生物的抗血栓形成活性上,主要是因为这些衍生物具有更强的抗血栓活性和更低的出血风险。此外,还报道了Hirudin/水蛭素的各种生物活性,包括伤口修复作用、抗纤维化作用、对糖尿病并发症的作用、抗肿瘤作用、抗高尿酸血症作用、对脑出血的作用等。因此,本文通过收集和总结近二十年来的出版物,系统地综述了水蛭素的药理活性、药代动力学、新制剂和衍生物以及毒性。此外,还讨论了水蛭素的临床应用、药理作用的潜在机制、剂量-效应关系以及新药研究的发展潜力,旨在为水蛭素在治疗相关疾病中的应用提供新思路。[1] 肾间质纤维化(RIF)常发生在许多慢性肾脏疾病(CKD)中。Hirudin/水蛭素现在用于治疗某些器官的纤维化。在这项研究中,我们验证了水蛭素在体内和体外对RIF的治疗作用及其潜在机制。体内RIF是单侧输尿管梗阻(UUO)模型,体外RIF是TGF-β诱导的肾小管上皮细胞。苏木精伊红(H&E)染色和Masson染色观察肾脏病理变化和肾纤维化。通过免疫组织化学方法检测肾组织中的α-SMA。通过ELISA法分析炎症因子。TUNEL法观察细胞凋亡情况。通过蛋白质印迹分析评估纤维化、上皮间质转化(EMT)和凋亡的相关蛋白。实验数据表明,水蛭素可减少UUO大鼠肾组织和TGF-β诱导的肾小管上皮细胞的纤维化、EMT、炎症和细胞凋亡。此外,水蛭素还降低了小鼠血清和TGF-β诱导的肾小管上皮细胞中胶原蛋白-I、FN、α-SMA、N-cad、slug、E-cad、IL-1β、IL-6和TNF-α的表达。UUO大鼠肾组织中凋亡相关蛋白(pro-capase3、pro-capase9、bcl2和bax)的表达也下调。综上所述,水蛭素通过抑制炎症、调节纤维化和ETM的相关蛋白以及减少肾小管上皮细胞的凋亡来抑制肾组织和肾小管上皮的纤维化。这些发现可能为RIF提供一种有效的治疗方法[2]。 水蛭素可减少UUO模型诱导的肾组织肾纤维化[2] 通过H&E染色观察肾组织的病理变化。UUO引起明显的肾损伤,伴有许多扩张和萎缩的小管。水蛭素干预部分缓解了上述肾损伤(图1A)。Masson染色显示,UUO模型诱导的肾组织存在明显的间质炎症和胶原沉积。免疫组织化学评估的UUO小鼠肾组织中α-SMA表达上调。水蛭素干预抑制了UUO小鼠α-SMA的上调和胶原沉积(图1B和C)。 水蛭素调节UUO模型诱导的肾组织纤维化相关蛋白和ECM的表达[2] 通过蛋白质印迹分析评估纤维化相关蛋白和ECM的表达,在UUO模型诱导的肾组织中,前者(胶原蛋白-I、FN和α-SMA)明显增加,而当UUO小鼠用水蛭素从10mg/kg治疗到15mg/kg时,前者减少(图2A)。ETM蛋白的表达如图2B所示。UUO模型诱导的肾组织中N-cad和slug的表达增加,E-cad的表达显著降低,水蛭素逐渐将N-cad、slug和E-cad的表达式从10mg/kg逆转到15mg/kg。 水蛭素降低UUO模型诱导的血清炎症因子的表达[2] ELISA法用于检测小鼠血清中IL-1β、IL-6和TNF-α的水平。UUO小鼠血清中IL-1β、IL-6和TNF-α水平上调。水蛭素治疗10 mg/kg至15 mg/kg的UUO小鼠可以降低血清中IL-1β、IL-6和TNF-α的水平(图3)。 水蛭素可减少UUO小鼠肾小管细胞的凋亡[2] TUNEL法显示UUO模型诱导肾组织肾小管细胞凋亡。当UUO小鼠用10mg/kg至15mg/kg的水蛭素治疗时,UUO小鼠肾组织中肾小管细胞的凋亡增加,减少(图4A)。因此,UUO小鼠肾小管细胞中切割的caspase3、切割的caspase-9和bax的表达上调,bcl2的表达下调。此外,水蛭素治疗后,UUO小鼠肾小管细胞中切割的caspase3、切割的caspase-9、bax和bcl2的表达被逆转。四组中pro-capase3和pro-capase9的表达没有变化(图4B)。 |
| 细胞实验 |
细胞培养和细胞诱导[2]
人HK-2细胞、小鼠IMCD3细胞和大鼠NRK-52E细胞购自ATCC。所有三种细胞系均在37°C、5%CO2的90%高糖DMEM和10%FBS中孵育。然后,用5ng/ml TGF-β处理三种细胞48小时,建立体外模型。 苏木精和伊红(H&E)染色[2] 将小鼠肾脏在4%多聚甲醛中固定48小时,然后包埋在石蜡中并切成切片。将切片放入二甲苯中20分钟,乙醇中5分钟,75%乙醇中5 min。随后,将切片放入苏木精中3分钟,用自来水洗涤,用乙醇脱水5分钟。最后,用中性胶密封的曙红对切片进行染色。 马森染色[2] 脱蜡步骤与HE染色相同。将切片在重铬酸钾中浸泡过夜,并用自来水洗涤。然后,将切片用苏木精染色3分钟,用自来水洗涤,用丽春红染色10分钟,并用自来水冲洗。随后,切片分别用磷钼酸溶液和苯胺蓝溶液染色3分钟,用中性胶密封。 免疫组织化学[2] 脱蜡步骤与HE染色相同。将切片在95°C下加热30-45分钟进行抗原修复。然后,将切片在室温下冷却,并在3%H2O2甲醇溶液中浸泡10分钟。将山羊血清加入切片中,在25°C下阻断10分钟。随后,将第一抗体、兔抗α-SMA抗体、第二抗体、生物素标记的山羊抗兔抗体和辣根过氧化物酶(HRP)标记的链霉抗生物素工作溶液依次加入切片中。然后,用DAB显色试剂盒对切片进行着色,用苏木精复染,然后用自来水冲洗。最后,用中性胶封闭切片,在显微镜下观察。 蛋白质印迹分析[2] 取肾组织和肾小管上皮细胞,用适当的RIPA裂解液裂解。然后高速离心后获得混合物的上清液,并使用BCA试剂盒检测不同组中的蛋白质浓度。根据测量的浓度计算每条泳道所需的蛋白质体积,并将蛋白质溶液煮沸10分钟。用10%SDS-PAGE分离蛋白质,并用PVDF膜转移。将PVDF膜在25°C下密封在脱脂牛奶中1小时。用于检测肾组织蛋白质的一级抗体是collgen-I、FN、α-SMA、E-cad、N-cad、slug、E-cad,裂解caspase3、裂解caspase9、pro-caspase3、pro-capase9、bcl2和bax。肾小管上皮细胞蛋白检测的主要抗体是collgen-I、FN、α-SMA、E-cad、N-cad和slug。PVDF膜与第一抗体在4°C下孵育过夜后,用TBST洗涤PVDF膜,并在25°C下用兔辣根过氧化物酶连接的IgG第二抗体处理1小时。加入ECL溶液后,将PVDF膜暴露在黑暗中,并用Bio-Rad凝胶记录系统扫描。最后,使用Quantity One软件分析每个条带的面积和灰度值。 酶联免疫吸附试验(ELISA)[2] 从小鼠眼球中采集血液,以5000rpm离心5分钟以分离血清。用ELISA试剂盒测定小鼠血清中IL-1β、IL-6和肿瘤坏死因子α(TNF-α)的水平。使用680型微孔板读数器获得450nm处的光密度(OD)值。 标记法 脱蜡步骤与HE染色相同。接下来,将切片与蛋白酶K在25°C下孵育15-30分钟。用PBS洗涤两次后,每个切片加入100μL TdT反应溶液,在37°C的湿箱中孵育1小时。每个切片用PBS洗涤三次,用50μL链霉抗生物素蛋白-荧光素-dUTP反应溶液在37°C下孵育1 h。最后,每个切片用50μL抗荧光淬灭剂密封30分钟,然后用荧光显微镜观察。 赫斯特染色[2] 脱蜡步骤与HE染色相同。将切片放置在振荡台上,用PBS洗涤两次。当切片被吸干后,在振荡台上用0.5ml Hoechst 33258处理5分钟。重复上述清洁程序。然后,将切片放在载玻片上,加入一滴防急冷密封溶液,并用干净的载玻片覆盖。荧光显微镜下可见凋亡细胞的蓝色核。 |
| 动物实验 |
Animal/Disease Models: Male balb/c (Bagg ALBino) mouse: with underwent unilateral ureteral ligation (UUO)[2]
Doses: 10 and 15 mg/kg Route of Administration: po (oral gavage); 10 and 15 mg/kg, one time/day for 21 days Experimental Results: diminished renal damages and suppressed the upregulation of α-SMA, collagen deposition in UUO mice. Increased the level of fibrosis (collagen-I, FN, α-SMA), N- cad, slug and E-cad in UUO mice. diminished the level of IL-1β, IL-6 and TNF-α, apoptosis of renal tubular cells in UUO mice. diminished the expression of inflammatory factors, the occurrence of EMT, the incidence of fibrosis and the apoptosis of TGF-β-induced renal tubular epithelial cell. In this experiment, mice underwent unilateral ureteral ligation (UUO) was adopted as the animal model. Forty male balb/c mice (25 g ± 3 g weight) were kept in SPF animal room with alternating light and dark for 12 h/time with humidity 60% and temperature 23 ± 3 °C and free access of water. The mice were randomly divided into four groups, including control group, UUO group, UUO + Hirudin (10 mg/kg) group and UUO + Hirudin (15 mg/kg) group, with ten mice in each group. All mice were weighed on the electronic balance to calculate the required anesthetic dose and then intraperitoneally injected with 2% pentobarbital (50 mg/kg). In the UUO group, mice were fixed on the rat board in the supine position and the abdominal skin of mice was disinfected with medical alcohol. The right abdominal incision (1.75 ± 0.25 cm) was taken and the peritoneum was opened. The right ureter was exposed and separated before its ligation with silk thread near the renal hilum and bladder. When no obvious bleeding appeared in the surgical field, the intestinal tract was rectified and the wound was sutured. In the UUO + Hirudin (10 mg/kg) group and UUO + Hirudin (15 mg/kg) group, mice were disposed with same surgery in UUO group and given the 10 mg/kg and 15 mg/kg Hirudin respectively by gavage once daily for duration of the study. The mice in the control group were not given any treatment. During the experiment, all the mice were free to eat and drink. On the 21th day after the operation, 1 ml blood was taken from eyeball of mice which then were sacrificed by cervical dislocation method and their right kidneys were obtained. [2] |
| 药代性质 (ADME/PK) |
Pharmacokinetics [1]
Pharmacokinetic studies of Hirudin and rH have been conducted in many species, including rats, rabbits, dogs, and human; the detailed pharmacokinetic parameters of these studies are shown in Table 2. Due to the polypeptide structure of hirudin, it is difficult to achieve the effective concentration by oral administration. Thus, hirudin is usually administered parenterally for high bioavailability. The pharmacokinetic behavior of hirudin among different animals tend to be the same. After intravenous administration, the kinetics of Hirudin manifests as a two-compartment open model (Markwardt et al., 1988b; Richter et al., 1988; Kaiser et al., 1990). The absorption half-life (t1/2α) suggested that hirudin can be rapidly distributed from the central compartment to the peripheral compartment. In addition, the elimination half-life (t1/2β) was about 1 h, which indicated that hirudin can be quickly excreted and metabolized. Besides, the bioavailability of Hirudin was almost 100% after subcutaneous administration, and the concentration-time curves illustrated that its plasma pharmacokinetics was consistent with a one-compartment model (Nowak et al., 1988). Compared with intravenous administration, the elimination half-life (t1/2) was obviously prolonged in both rats (2.1 h) and dogs (3.03 h). Similar to natural hirudin, rH could be rapidly distributed into the extravascular compartment with t1/2α from 0.08 to 0.25 h. In addition, the elimination phase half-lives were relatively longer after intravenous administration. Remarkably, most of the administered hirudin and rH could be eliminated through kidney in active form in dogs (Nowak et al., 1988). Moreover, in healthy volunteers, renal clearance and degradation accounted for 90% of systemic clearance (Greinacher and Lubenow, 2001). In addition to subcutaneous administration and intravenous administration, other routes of rH administration have been investigated. The in vivo course of rHV2 in rats fitted to the one-compartment model after intranasal administration of rHV2 spray with the relative bioavailability of 28.53% (Zhang et al., 2006). Furthermore, Zhang et al. reported that the relative bioavailability (FR) of rHV2 liposome and rHV2 saline solution after intranasal administration were 12.36 and 1.83%, respectively. These results demonstrated that the pharmacokinetics and bioavailability of rH were greatly affected by the dosage form (Zhang et al., 2007). Moreover, Liu et al. compared the pharmacokinetics of rH in rats administered through four different routes (intratracheal, buccal, nasal and rectal) and found that the pulmonary route was more suitable for systemic delivery of rHV2 than other three routes (Liu et al., 2005). The Preparations of Hirudin [1] In order to reduce the adverse reactions and improve the bioavailability of Hirudin, various types of preparations have been reported in recent years, such as micelles, nanoparticles, TiO2 nanotube systems, polyamides dendrimer, and so on. Remarkably, the characteristics of these novel preparations mainly focus on prolonging circulation, thrombus targeting, continuous drug delivery, and specific disease targeting. Prolonging Circulation [1] Hirudin can be rapidly distributed into the intercellular space after intravenous injection and has a short half-life; repeated injections are needed to maintain its therapeutic effect. However, several drawbacks (such as high price and bleeding risk) will be brought about after repeated injections, which greatly limited the clinical application of hirudin. The bovine serum albumin (BSA) nanoparticles are considered to be a promising carrier to address those problems (Green et al., 2006). In 2015, hirudin-BSA nanoparticles have been successfully synthesized and characterized by a desolvation technique. These nanoparticles possess good encapsulation and certain sustained-release capacity, which could control the release of hirudin to prolong the antithrombotic effect, suggesting that hirudin-BSA nanoparticles might be applied in clinic therapy for thrombosis. However, the pharmacokinetics and safety of these novel preparations need to be further studied (Jing et al., 2016). In addition to nanoparticles, polydopamine fitted TiO2 nanotube systems were also exploited to extend the release of hirudin. In 2018, Yang et al. reported that PDA fitted TiO2 nanotube systems could prolong the release of bivalirudin and improve the hemocompatibility in vitro and in vivo. Besides, the systems could effectively reduce the formation of thrombosis by inhibiting the denaturation as well as adhesion of platelets, fibrinogen, and other blood components (Yang et al., 2018). Although Hirudin could exert platelet aggregation inhibitory effect after structurally modified with platelet-targeting peptides such as RGD, the short half-life of this derivative is still a severe problem. Polyion complex (PIC) micelles have attracted extensive attention in recent years due to its complementary and unique features (Yang et al., 2009), which enables the long circulation of drugs in vivo. Recombinant hirudin variant 2 (rHV2)-loaded PIC micelles, consisting of methoxy poly (ethylene glycol)-grafted-chitosan (mPEG-g-chitosan) and Arg-Gly-Asp conjugated poly (ethylene glycol)-grafted-chitosan (RGD-PEG-g-chitosan), were prepared by Wang et al. in 2010. These rHV2-loaded PIC micelles, with the mean size of 41.9 ± 1.8 nm and the encapsulation efficiencies of 81.08 ± 0.85%, could prolong the mean retention time of rHV2 and specifically bind to platelets. Besides, efficient anticoagulant effects and platelet aggregation inhibition effects were observed in these micelles, which indicated that RGD-PIC micelles could achieve the purpose of platelet-targeted delivery and long circulation of rHV2 (Wang et al., 2010). |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity [1]
The toxic studies of Hirudin and its derivatives have been performed in several animal experiments. Previous studies revealed that after subcutaneous injection of hirudin 100 mg/kg for 8 days, the hemorrhage of pleura, pia meninges and peritoneum occurred in rats. In addition, daily doses of hirudin from 1 mg/kg to 5 mg/kg resulted in a dose-dependent increase in bleeding propensity in sub-chronic toxicity studies in rats and dogs for up to 3 months. It's worth noting that the formation of antibodies could be observed after the administration of extremely high doses of hirudin in dogs, but these antibodies did not neutralize the effect of hirudin (Nowak, 2002). Interestingly, such antibodies were also detected in humans during long-term administration of hirudin (Eichler et al., 2000), however, the antibody-bound hirudin could exert antithrombin property as before with the longer half-life, indicating that these antibodies might be a form of Hirudin storage. Moreover, the lethal dose (LD 50) of lyophilizing hirudin powder was determined to be over 10.0 g/kg when administered via orally administration, and no significant difference was observed in micronucleus rate and sperm malformation rate between hirudin groups (2.5, 5.0, and 10.0 g/kg) and control group (Huang et al., 2010). Furthermore, lyophilizing hirudin powder exhibited no maternal toxicity, embryo toxicity, and teratogenicity in rats at the dosages of 312.5, 1250, and 5000 mg/kg (Huang et al., 2011). Besides, the toxicity of rH has been investigated in some reports. Lu et al. discovered that rH (1.0, 3.0, 6.0 mg/kg) could significantly prolong the clotting time, thrombin time, and activated partial thromboplastin time in Macaca mulatta, however, these effects could be automatically reversed at 24 h after administration (Lu et al., 2004). These studies proved that hirudin rarely causes toxicity despite its minor bleeding side effect, which can be used for the prevention and treatment of more diseases with a broad development prospect. 72941487 mammal (species unspecified) LD50 intravenous >50 mg/kg Zhongguo Yaoxue Zazhi. Chinese Pharmacuetical Journal., 26(396), 1991 |
| 参考文献 |
|
| 其他信息 |
Single-chain polypeptides of about 65 amino acids (7 kDa) from LEECHES that have a neutral hydrophobic N terminus, an acidic hydrophilic C terminus, and a compact, hydrophobic core region. Recombinant Hirudins lack tyr-63 sulfation and are referred to as 'desulfato-hirudins'. They form a stable non-covalent complex with ALPHA-THROMBIN, thereby abolishing its ability to cleave FIBRINOGEN.
In the past few decades, notable findings of Hirudin have been gained, precisely including the clinical application, development of derivatives, exploration of novel pharmacological activities, and investigation of new preparations. Successful Application of Derivatives of Hirudin in Clinic: With the development of genetic engineering technology, the producing problems of natural Hirudin has been successfully solved, which greatly promoted the successfully application of lepirudin, desirudin and bivalirudin in clinic. Lepirudin has been approved as an anticoagulant for patients with heparin-induced thrombocytopenia (HIT), with the usual dose of 0.4 mg/kg IV bolus followed by 0.15 mg/kg/h IV infusion (Greinacher and Lubenow, 2001). It could reduce HIT related adverse events by 72%, providing not only effective anticoagulant therapy, but also rapid platelet recovery in HIT patients. Noticeably, the bolus and infusion rates should be scheduled according to the serum creatinine values of patients (Greinacher et al., 1999). Desirudin is used to preventing deep venous thrombosis (DVT) in patients undergoing total hip replacement (THR) and total knee replacement (TKR) surgery by subcutaneously (SC) injection. Previous studies reported that desirudin was superior in preventing VTE in patients undergoing elective hip replacement surgery at the dose of 15 mg (Q12H, SC) compared with low-dose unfractionated heparin and low-molecular-weight heparin (Eriksson et al., 1997) (Bergese et al., 2013). Another direct thrombin inhibitor, bivalirudin, is effective on preventing thromboembolic complications. It was reported that administrating bivalirudin as a 1.0 mg/kg IV bolus, followed by 2.5 mg/kg/h by IV infusion for 4 h early in the trial; later, lowering the bolus to 0.75 mg/kg, followed by a 1.75 mg/kg/h infusion over 4 h achieved procedural and clinical success in 98 and 96% of the patients, respectively (Sun et al., 2017). Additionally, bivalirudin is also being studied in phase II and III multicenter trials as an alternative anticoagulant for both on-pump and off-pump cardiac surgery for patients with or without HIT (Joseph et al., 2014). [1] Future Perspectives: Although the studies of Hirudin have referred to pharmacy, pharmacology, preparation and clinical application, we find that there is still somewhat inadequate, especially studies of the dose-effect relationship, toxicity and safety of its derivatives and preparations. In this section, we focus on potential future directions for research on hirudin. Given the multiple pharmacological activities nature of Hirudin, elaborating its dose-effect relationship is beneficial to the determination of doses for clinical treatment of different diseases in the future. Remarkably, hirudin exhibited different activities and even opposite biological effects at different concentrations. For instance, the expression of VEGF was increased when hirudin was used to treat necrosis of skin flap, but VEGF was inhibited in hirudin–treated diabetic complications and tumor. This difference may be caused by the different doses of hirudin used in these two studies. At low concentrations (1 and 4 ATU/ml), hirudin can promote cell proliferation and micro-angiogenesis by upregulating VEGF, which is quite important for wound repair. However, with higher concentration (7 ATU/ml), the promoting effect of hirudin on the VEGF/Notch signaling pathway gradually decreased and turned into an inhibitory effect. Accordingly, the expression of VEGF decreased, indicating that high concentration of hirudin can inhibit the growth of cells and exert the anti-inflammatory activity, which is conducive to the treatment of diabetic complications and tumor. Likewise, the effect of hirudin on ERK1/2 pathway is also bidirectional. At the dosage of 2ATU, Hirudin could significantly elevate the expression level of ERK1/2 phosphorylation; activated ERK promoted the transcription of VEGF signaling molecule by regulating the expression of AP-1 and c-Jun, which facilitated the neovascularization of ischemic skin flap rats. However, at other dosages (20, 40, and 80 μg/ml), hirudin could down-regulate the expression of ERK1/2 phosphorylation to participate in the treatment of fibrosis. Detailed information on the pharmacological activity, route of administration, effective dose and molecular mechanism of hirudin is provided in Table 4. It is worth noting that besides concentrations, different routes of administration and the animal models or cells used in different studies may contribute to the differences in bioactivities as well. Therefore, more in vivo researches are quite needed to clarify the dose-effect relationship of hirudin. [1] Although Hirudin has been successfully used for thrombosis therapy in clinic, its short half-life and the bleeding risk remain limiting factors, further researches may focus on the structural modifications. Perhaps, fusing recognition peptides identified from FXIa and FXa with these drugs might be a promising method to reduce bleeding risk. In addition, the studies associated with the derivatives and preparations of hirudin are only at the preliminary stage and further studies should be conducted to reveal the actions, toxicity and potential mechanisms. In short, Hirudin has a wide range of pharmacological activities and enormous potential for clinic application, which is worthy of more in-depth and comprehensive study. [1] Hirudin has a strong inhibitory effect on thrombin with anticoagulant, antithrombotic, antitumor and anti-fibrosis functions. Hirudin reduced the deposition of fibrin-related antigens in glomerulus, decreased the proliferation of glomerular mesangial cells and glomerular sclerosis and reduced the proteinuria and low protein blood to improve the renal function. Hirudin may downregulate the expression of TGF-β and α-SMA, and reduce the proliferation and activation of fibroblast growth factors and fibroblast phenotype transformation, thereby preventing and treating renal interstitial fibrosis. In this study, hirudin decrease the inflammation and the occurrence of EMT in renal tubular epithelial cells to inhibit the fibrosis and apoptosis of renal tubular epithelial cells. Here, hirudin suppressed the fibrosis in renal tissues and renal tubular epithelial cells by inhibiting the inflammation, regulating the fibrosis-related proteins and ECM and decreasing the apoptosis of renal tubular epithelial cells. However, natural products often have the potential liver injury. When natural products has been studied to have a protective effect on kidney injury, we also investigate the effects of natural products on liver in the future study.[2] |
| 分子式 |
C68H94F3N13O27
|
|---|---|
| 分子量 |
1582.5401
|
| 精确质量 |
1467.64
|
| CAS号 |
113274-56-9
|
| 相关CAS号 |
Hirudin (54-65) (TFA)
|
| PubChem CID |
16138839
|
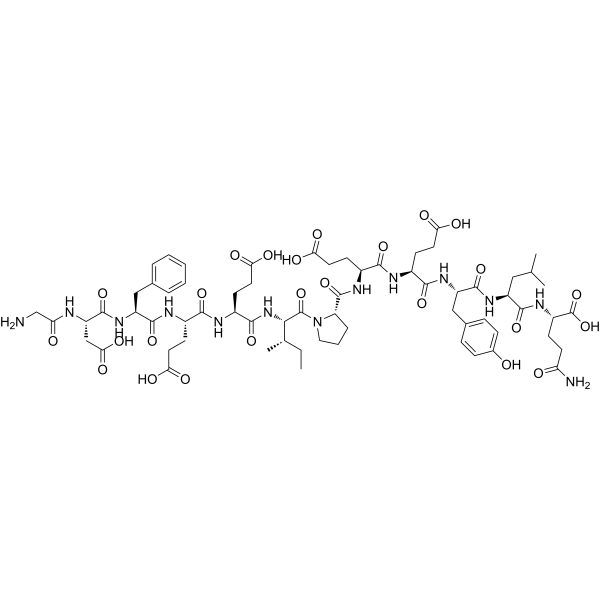
| 序列 |
H-Gly-Asp-Phe-Glu-Glu-Ile-Pro-Glu-Glu-Tyr-Leu-Gln-OH; glycyl-L-alpha-aspartyl-L-phenylalanyl-L-alpha-glutamyl-L-alpha-glutamyl-L-isoleucyl-L-prolyl-L-alpha-glutamyl-L-alpha-glutamyl-L-tyrosyl-L-leucyl-L-glutamine
|
| 短序列 |
GDFEEIPEEYLQ;
H-GDFEEIPEEYLQ-OH
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
1913.6±65.0 °C at 760 mmHg
|
| 闪点 |
1111.1±34.3 °C
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
| 折射率 |
1.587
|
| LogP |
-0.19
|
| tPSA |
624.45
|
| 氢键供体(HBD)数目 |
19
|
| 氢键受体(HBA)数目 |
26
|
| 可旋转键数目(RBC) |
47
|
| 重原子数目 |
104
|
| 分子复杂度/Complexity |
3050
|
| 定义原子立体中心数目 |
12
|
| SMILES |
FC(C(=O)O[H])(F)F.O=C([C@]([H])([C@@]([H])(C([H])([H])[H])C([H])([H])C([H])([H])[H])N([H])C([C@]([H])(C([H])([H])C([H])([H])C(=O)O[H])N([H])C([C@]([H])(C([H])([H])C([H])([H])C(=O)O[H])N([H])C([C@]([H])(C([H])([H])C1C([H])=C([H])C([H])=C([H])C=1[H])N([H])C([C@]([H])(C([H])([H])C(=O)O[H])N([H])C(C([H])([H])N([H])[H])=O)=O)=O)=O)=O)N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(N([H])[C@]([H])(C(N([H])[C@]([H])(C(N([H])[C@]([H])(C(N([H])[C@]([H])(C(N([H])[C@]([H])(C(=O)O[H])C([H])([H])C([H])([H])C(N([H])[H])=O)=O)C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])=O)C([H])([H])C1C([H])=C([H])C(=C([H])C=1[H])O[H])=O)C([H])([H])C([H])([H])C(=O)O[H])=O)C([H])([H])C([H])([H])C(=O)O[H])=O
|
| InChi Key |
ZNEKMMIWGPUTGR-SQJOKQRMSA-N
|
| InChi Code |
InChI=1S/C66H93N13O25/c1-5-34(4)55(78-59(96)41(21-26-53(89)90)71-56(93)38(18-23-50(83)84)72-61(98)44(29-35-10-7-6-8-11-35)77-63(100)46(31-54(91)92)69-49(82)32-67)65(102)79-27-9-12-47(79)64(101)73-40(20-25-52(87)88)57(94)70-39(19-24-51(85)86)58(95)76-45(30-36-13-15-37(80)16-14-36)62(99)75-43(28-33(2)3)60(97)74-42(66(103)104)17-22-48(68)81/h6-8,10-11,13-16,33-34,38-47,55,80H,5,9,12,17-32,67H2,1-4H3,(H2,68,81)(H,69,82)(H,70,94)(H,71,93)(H,72,98)(H,73,101)(H,74,97)(H,75,99)(H,76,95)(H,77,100)(H,78,96)(H,83,84)(H,85,86)(H,87,88)(H,89,90)(H,91,92)(H,103,104)/t34-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,55-/m0/s1
|
| 化学名 |
(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-1-[(2S,3S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[(2-aminoacetyl)amino]-3-carboxypropanoyl]amino]-3-phenylpropanoyl]amino]-4-carboxybutanoyl]amino]-4-carboxybutanoyl]amino]-3-methylpentanoyl]pyrrolidine-2-carbonyl]amino]-4-carboxybutanoyl]amino]-4-carboxybutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-methylpentanoyl]amino]-5-oxopentanoic acid
|
| 别名 |
Hirudin (54-65) (desulfated); 113274-56-9; Hirudin (54-65); Hirudin (54-65; Hirudin (54-65) (desulfated) trifluoroacetate salt; Hirudin(54-65)(desulfated); CHEMBL411059;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.6319 mL | 3.1595 mL | 6.3190 mL | |
| 5 mM | 0.1264 mL | 0.6319 mL | 1.2638 mL | |
| 10 mM | 0.0632 mL | 0.3159 mL | 0.6319 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。