| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| Other Sizes |
|
| 靶点 |
HCV polymerase (IC50 = 0.31 μM; Ki = 52.3 nM)
|
|---|---|
| 体外研究 (In Vitro) |
前药 IDX184 以肝脏为靶点,含有核苷酸 2'-MeG-MP。丙型肝炎病毒核苷酸聚合酶被 IDX184(一种肝脏靶向药物)抑制 [1]。与母体核苷相比,IDX184 是第二代口服生物可利用的核苷酸前药,旨在提供增强的抗 HCV 功效和安全性。在 HCV 复制子测试中,IDX184 在所有修饰核苷(2' 或 4';EC50=4-6 μM)中具有最高效力(EC50=0.3-0.45)。它对感染 JFH-1 的病毒也有效(EC50=0.06-0.11 μM)。在所研究的细胞系中,IDX184没有引起任何毒性(CC50>100μM)[2]。
|
| 体内研究 (In Vivo) |
IDX184是一种核苷酸前药,旨在促进2'-甲基鸟苷(2'-MeG)活性三磷酸在肝脏中的形成,2'-MeG是丙型肝炎病毒(HCV)的一种有效和特异性聚合酶抑制剂。在本研究中,8名健康受试者按顺序口服5、10、25、50、75和100 mg IDX184单次递增剂量,按6:2随机分组,分为活性/安慰剂组。在给药后120小时内进行血浆和尿液药代动力学采样。吸收后,IDX184迅速从血浆中消失,平均半衰期(t(1/2))约为1小时,而2′-MeG的血浆浓度逐渐升高。与肝脏靶向方法一致,IDX184和2'-MeG的血浆暴露量较低,也与剂量相关:IDX184的平均最大浓度范围为1.1至17 ng/ml, 2'-MeG的平均最大浓度范围为1.7至19 ng/ml,曲线下的平均总面积范围为1.2至22.7和17.3至334 ng·h/ml。25 ~ 100 mg给药24 h后平均2′- meg血浆浓度为0.6 ~ 3 ng/ml。25毫克及以上剂量的平均2'-MeG t(1/2)值为18至43小时。不变的IDX184和2'-MeG的平均累积尿排泄量分别为给药剂量的0.2%和12 - 20%。IDX184安全且耐受性良好;未观察到严重不良事件(sae)、剂量依赖性不良事件(ae)或剂量限制性毒性。不良事件和实验室异常的发生率较低,在接受IDX184或安慰剂的受试者中相似。所有ae均为轻度至中度,并在研究结束时消退。良好的安全性和药代动力学特征支持IDX184在hcv感染患者中的进一步临床评价。[1]
|
| 动物实验 |
In HCV-infected chimpanzees receiving 10 mg/kg/day of IDX184 as a single agent for 4 days, the viral response at the end of treatment correlated significantly with 2′-MeG exposure but not with IDX184 exposure. Trough concentrations of 2′-MeG ranging from 2 to 8 ng/ml were associated with 1 to 4 log10 reductions in HCV RNA (6, 7). In the current study, plasma concentrations of 2′-MeG remained quite sustained, i.e., still above 2 ng/ml 24 h after a single dose of 50 to 100 mg IDX184. Steady-state trough concentrations were predicted to be around 10 ng/ml after repeat once-per-day (QD) dosing (data not shown). In light of the pharmacokinetic/pharmacodynamic (PK/PD) relationship established in the chimpanzees, a dose-dependent viral response can be anticipated with IDX184 treatment in HCV-infected patients [1].
|
| 药代性质 (ADME/PK) |
Plasma pharmacokinetics.[1]
IDX184 is a prodrug that delivers the monophosphate of the nucleoside 2′-MeG in the liver. Both the parent, IDX184, and its nucleoside metabolite, 2′-MeG, were monitored in the blood. Mean (+standard deviation [SD]) plasma concentration-time curves of IDX184 (left panel) and 2′-MeG (right panel) after administration of single doses of IDX184 escalating from 5 to 100 mg are depicted in Fig. 2. Summary pharmacokinetic parameters are presented in Table 2. Following oral administration of a single dose under fasted conditions in healthy subjects, IDX184 was rapidly absorbed, and the Tmax corresponded in most subjects to the first sampling time, with a cohort median value of 0.25 to 0.49 h regardless of the administered doses. The level of plasma exposure to IDX184 was low and proportional to administered doses, with a cohort mean Cmax and AUC0-∞ ranging from 1.12 to 17.3 ng/ml and 1.19 to 22.7 ng·h/ml, respectively, as doses escalated from 5 to 100 mg. The pharmacokinetic dose proportionality of IDX184 in the studied dose range was evaluated by regression analyses of log-transformed parameters of exposure and doses. The model estimate of the slope was close to unity for Cmax (b = 0.98; 95% CI = 0.76 to 1.21) and AUC0-∞ (b = 1.01; 95% CI = 0.85 to 1.17). The plasma kinetic profile of IDX184 exhibited a steep disposition phase, with plasma concentrations quickly falling below the limit of quantitation (0.1 ng/ml) with a short yet consistent mean t1/2 of 0.58 to 1.06 h across doses. Cohort mean CL/F and Vd/F, normalized to body weight, were 49.0 to 92.1 liters/h/kg and 67.8 to 122 liters/kg, respectively, and were independent of administered doses of IDX184. The rapid elimination of IDX184 from plasma was accompanied by the gradual appearance of 2′-MeG. Similar to results for the parent drug, the plasma concentrations of 2′-MeG were also low and reached a maximum at a cohort median Tmax of 4.00 to 6.00 h across doses. While mean Cmax and mean C24 values of 2′-MeG increased 10-fold, from 1.74 to 18.6 ng/ml and 0.25 to 2.88 ng/ml, respectively, with doses in the 5- to 50-mg range, these parameters became less dose proportional at higher doses (Table 2). The values of slope b (95% CI) resulting from dose-proportionality analyses were 0.76 (0.60 to 0.92) for Cmax and 0.84 (0.65 to 1.03) for C24. Mean Cmax and mean C24 values of 2′-MeG with 50- to 100-mg doses of IDX184 were 14.6 to 18.6 ng/ml and 2.23 to 2.88 ng/ml, respectively. In contrast, the mean AUC0-∞ of 2′-MeG had a 20-fold increase, from 17.3 to 334 ng·h/ml, as the dose increased 20-fold, and the values were shown to be dose proportional in the studied dose range (b = 1.04; 95% CI = 0.88 to 1.19). The mean elimination t1/2 of 2′-MeG, whose estimation depends on the actual observed portion of the elimination phase (concentration > LOQ), ranged from 5.42 to 12.2 h for doses up to 10 mg and from 18.0 to 42.5 h for doses of ≥25 mg. The cohort mean ratios of IDX184 to 2′-MeG AUC0-∞ on a molar basis were low (<4%) and remained independent of the amount of dose administered, indicating a near-complete and unsaturated biotransformation of IDX184 in the studied dose range. Urine excretion.[1] The amounts of parent IDX184 and its nucleoside metabolite 2′-MeG excreted in urine were monitored during a 120-h interval after administration in all subjects. Urine IDX184 could only be measured up to 12 h postdose except for one sample, where IDX184 was observed up to 24 h. Cumulative urine excretion of IDX184, which increased with increasing doses, remained consistently low across cohorts, representing only 0.2% of administered doses (Table 3). In contrast, urine 2′-MeG was quantifiable in all fractions during the entire 120-h postdose collection period. In general, the cumulative amount of 2′-MeG excreted in urine increased as the dose was escalated (Table 3). The cumulative amount of 2′-MeG excreted in urine was higher than that of IDX184, representing, on a molar basis, 12 to 20% of administered doses. Near-maximum cumulative urine excretion of 2′-MeG was reached between 48 and 72 h, and more than half of the total amount of 2′-MeG excreted was recovered within the first 24 h. Renal clearance (CLR) was independent of the amount of dose administered and remained consistent across cohorts, with a cohort mean of 123 to 177 ml/min for IDX184 and 271 to 322 ml/min for 2′-MeG. |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety and tolerability. [1]
Single-dose IDX184 was well tolerated. There were no SAEs or dose-limiting toxicities. The most common AE was dizziness, which occurred in 2 of 36 subjects (5.6%) exposed to IDX184 and 3 of 12 subjects (25%) exposed to a placebo. Other, less-frequent AEs observed in both placebo and active groups included dermatitis contact, dysmenorrhoea, fatigue, and headache. AEs reported were all mild or moderate in intensity and resolved by the end of the study. There were no discernible patterns in AEs between treatment groups. Laboratory parameters were stable over time for all treatment groups. No clinically meaningful changes were observed in vital sign measurements, physical examination findings, or ECG parameters. |
| 参考文献 | |
| 其他信息 |
Currently, ethical concerns and technical challenges prevent direct assessment in humans of hepatic intracellular IDX184 and related phosphorylated forms of 2′-MeG. While liver biopsy specimens were not obtained in this study, the extent of conversion from IDX184 to 2′-MeG-MP and subsequent phosphates may nevertheless be approximated by the ratio of plasma exposure of parent IDX184 to 2′-MeG. Despite a 20-fold change in dose, the mean molar ratio of AUC0-∞ of IDX184 to 2′-MeG, ranging from 2.2 to 3.8%, was low and consistent across doses, suggesting a near-complete conversion which was not saturated at the highest IDX184 dose studied.
Parent IDX184 and its metabolite 2′-MeG were excreted in urine. Although no formal statistical analyses were performed, the amounts of both entities recovered in urine appeared to be dose related. Urinary excretion of IDX184 was limited, whereas cumulative urine excretion of 2′-MeG was substantial, accounting for approximately 12 to 20% of the administered dose. Renal clearance, in particular for 2′-MeG, was greater than the normal glomerular filtration rate of 80 to 120 ml/min, indicating the involvement of active components in renal elimination. In summary, single oral doses of 5 mg to 100 mg of IDX184 were safe and well tolerated by the healthy subjects in this study. Following oral administration, plasma IDX184 and 2′-MeG concentrations were low, consistent with IDX184 being a liver-targeted prodrug. The favorable safety and pharmacokinetic profiles of IDX184 support further clinical evaluation of IDX184 in HCV-infected patients.[1] |
| 分子式 |
C25H35N6O9PS
|
|---|---|
| 分子量 |
626.62
|
| 精确质量 |
626.192
|
| 元素分析 |
C, 47.92; H, 5.63; N, 13.41; O, 22.98; P, 4.94; S, 5.12
|
| CAS号 |
1036915-08-8
|
| PubChem CID |
135565589
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
1.892
|
| tPSA |
259.25
|
| 氢键供体(HBD)数目 |
6
|
| 氢键受体(HBA)数目 |
13
|
| 可旋转键数目(RBC) |
14
|
| 重原子数目 |
42
|
| 分子复杂度/Complexity |
1060
|
| 定义原子立体中心数目 |
4
|
| InChi Key |
FGHMGRXAHIXTBM-TWFJNEQDSA-N
|
| InChi Code |
InChI=1S/C25H35N6O9PS/c1-24(2,13-32)22(35)42-10-9-38-41(37,28-11-15-7-5-4-6-8-15)39-12-16-18(33)25(3,36)21(40-16)31-14-27-17-19(31)29-23(26)30-20(17)34/h4-8,14,16,18,21,32-33,36H,9-13H2,1-3H3,(H,28,37)(H3,26,29,30,34)/t16-,18-,21-,25-,41?/m1/s1
|
| 化学名 |
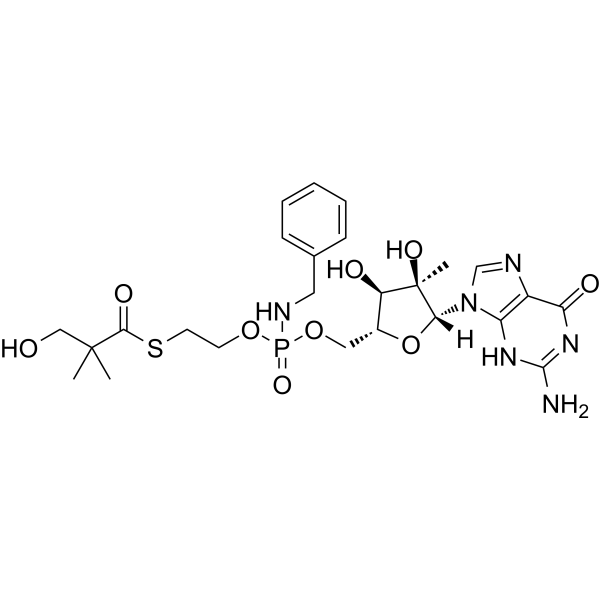
S-[2-[[(2R,3R,4R,5R)-5-(2-amino-6-oxo-1H-purin-9-yl)-3,4-dihydroxy-4-methyloxolan-2-yl]methoxy-(benzylamino)phosphoryl]oxyethyl] 3-hydroxy-2,2-dimethylpropanethioate
|
| 别名 |
IDX-184; IDX184; 1036915-08-8; Guanosine, 2'-C-methyl-, 5'-(2-((3-hydroxy-2,2-dimethyl-1-oxopropyl)thio)ethyl N-(phenylmethyl)phosphoramidate); 4W44B4S9OC; S-[2-[[(2R,3R,4R,5R)-5-(2-amino-6-oxo-1H-purin-9-yl)-3,4-dihydroxy-4-methyloxolan-2-yl]methoxy-(benzylamino)phosphoryl]oxyethyl] 3-hydroxy-2,2-dimethylpropanethioate; UNII-4W44B4S9OC; SCHEMBL3132403; IDX 184
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~250 mg/mL (~398.97 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5959 mL | 7.9793 mL | 15.9586 mL | |
| 5 mM | 0.3192 mL | 1.5959 mL | 3.1917 mL | |
| 10 mM | 0.1596 mL | 0.7979 mL | 1.5959 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。