| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
HCAR1/hydroxycarboxylic acid receptor 1; Endogenous Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
乳酸和HCAR1靶向在葡萄膜黑色素瘤细胞系中发挥相反的作用
抑制乳酸摄取诱导葡萄膜黑色素瘤生长。
乳酸治疗增加葡萄膜黑色素瘤中HCAR1和乳酸转运蛋白。
乳酸重塑葡萄膜黑色素瘤代谢,增加参与线粒体代谢的基因mRNA水平。
补充乳酸可提高葡萄膜黑色素瘤细胞的常染色质率和静止性[1]。
葡萄膜黑色素瘤(Uveal melanoma, UM)是成人最常见的原发性眼内癌,是预后较差的肿瘤之一。最近,肿瘤代谢物乳酸盐的作用变得越来越有吸引力,因为它作为羟基羧酸受体1 (HCAR1)激活剂,作为诱导赖氨酸残基乳酸化的表观遗传调节剂,当然,作为糖酵解的最终产物,弥合了糖酵解和氧化磷酸化之间的差距。本研究的目的是利用已知的HCAR1和乳酸转运蛋白调节剂,在UM细胞系(92.1)中剖析乳酸作为代谢物或信号分子的作用。我们的研究结果表明,乳酸(20 mM)导致细胞增殖和迁移显著减少,并将细胞代谢转向氧化磷酸化。这些结果与UM细胞的常染色质含量增加和静止相结合。我们进一步在临床环境中发现,乳酸转运体MCT4和HCAR1的增加与UM的纺锤形组织学类型有关。总之,我们的研究结果表明,乳酸代谢可能作为UM进展的预后标记物,并可能作为潜在的治疗靶点。 |
| 体内研究 (In Vivo) |
乳酸通过CD8+ T细胞在多种肿瘤模型中促进抗肿瘤免疫。
乳酸治疗增加肿瘤浸润性CD8+ T细胞。
乳酸治疗增加MC38肿瘤中干细胞样CD8+ T细胞群。
乳酸增加体外扩增过程中TCF-1的表达,减少CD8+ T细胞的凋亡。
乳酸通过表观遗传调控诱导T细胞的干细胞性。
乳酸预处理的CD8+ T细胞过继转移在体内实现了有效的肿瘤生长抑制。[2]
乳酸是葡萄糖分子糖酵解代谢产生的关键代谢物,也是许多细胞类型的主要碳燃料来源。在肿瘤免疫微环境中,乳酸对癌症和免疫细胞的影响可能非常复杂且难以破译,这进一步被糖酵解的副产物酸性质子所混淆。本研究表明,乳酸能够增加CD8+ T细胞的干性,增强抗肿瘤免疫。皮下注射乳酸钠而非葡萄糖对移植MC38肿瘤小鼠产生CD8+ T细胞依赖性肿瘤生长抑制作用。单细胞转录组学分析显示,肿瘤内CD3+细胞中表达CD8+ T细胞的干细胞样tcf -1的比例增加,这一表型通过体外乳酸处理T细胞证实。从机制上讲,乳酸抑制组蛋白去乙酰化酶活性,导致Tcf7超级增强子位点H3K27乙酰化增加,导致Tcf7基因表达增加。体外乳酸预处理的CD8+ T细胞在过继转移到荷瘤小鼠后有效抑制肿瘤生长。我们的研究结果为乳酸在抗肿瘤免疫中的内在作用提供了证据,该作用独立于乳酸的ph依赖效应,并可能推进癌症免疫治疗。[2] |
| 细胞实验 |
细胞增殖的实时监测[1]
xCELLigence实验使用如前所述的实时细胞分析(RTCA)双板(DP)仪器进行。简单地说,通过细胞滴定和生长实验确定了最佳的播种数量。播种至最佳细胞数(3000个/孔)后,分别用乳酸、AZD3965、3,5- dhba和3- oba处理细胞,每15 min自动监测24 h。 药物治疗对细胞迁移的影响[1] 采用创面愈合实验检测细胞迁移。简单地说,细胞在24孔培养皿中播种并培养至融合。在这一阶段,在需要的地方加入乳酸、AZD3965、3,5- dhba或3- oba,并用200 mL微管尖端刮擦细胞培养物。分别于0、24、48 h检测创面闭合情况。利用ImageJ v1.37软件,以不同间隔测量创面未覆盖面积。 免疫细胞化学分析[1]< br > 免疫细胞化学按先前报道进行。简单地说,根据制造商的说明,用200 nM MitoTracker Red CMXRos探针在37°C下对线粒体进行30分钟的染色。细胞在37℃下用染料处理30分钟,30分钟后去除染料。在此阶段,细胞在磷酸盐缓冲盐水(PBS)中洗涤3次,以去除未结合的探针。根据制造商的说明,细胞核用NucBlue染色(每mL两滴),在37℃下染色15分钟。最后,用20 mM乳酸处理细胞。为了获取图像,我们使用了Operetta,将细胞保存在37°C,并在处理后24 h捕获图像。收集的数据用Harmony软件进行分析。 |
| 动物实验 |
Tumor growth and treatment [2]
C57BL/6 J mice were inoculated with 1 × 106 MC38 tumor cells, or 1.5 × 105 TC-1 tumor cells or 1.5 × 105 B16F10 tumor cells on the right flank on day 0. Same volume (2 mL) of isotonic sodium lactate (150 mM, pH 7.4) or glucose solution (278 mM, pH 7.4) was administered subcutaneously under the dorsal skin near the neck. For MC38 model, animals were intraperitoneally (i.p.) treated with anti-PD-1 (10 mg/kg, day 7 and 10) in combination with glucose or sodium lactate (s.c., 5 g/kg or 1.68 g/kg, respectively) daily, beginning on day 8. For TC-1 tumor model, animals were treated with anti-PD-1 (i.p. 10 mg/kg, day 11 and 14) or PC7A vaccine (s.c. 0.5 μg E7 peptide, day 11 and 16) in combination with glucose or sodium lactate (s.c., 5 g/kg or 1.68 g/kg, respectively) daily, beginning on day 12. For B16F10 tumor model, animals were treated with anti-PD-1 (i.p. 10 mg/kg, day 5 and 8) in combination with glucose or sodium lactate (s.c., 5 g/kg or 1.68 g/kg, respectively) daily, beginning on day 6. For immune cell depletion assay, anti-CD8 antibodies, anti-CD4 antibodies or anti-NK1.1 were administrated every three days during the treatment (i.p. 10 mg/kg). For single cell analysis and in vivo flow cytometry analysis in MC38 tumor model, animals were intraperitoneally (i.p.) treated with anti-PD-1 (10 mg/kg, day 14 and 17) in combination with or without sodium lactate (s.c., 1.68 g/kg) daily, beginning on day 15. Tumor and tumor draining lymph nodes were collected on day 20 for analysis. Tumor volumes were measured with a caliper by the length (L), width (W) and height (H) and calculated as tumor volume = L×W×H/2. Animals were considered dead when tumor volume reached > 1500 mm3. Tumor free C57BL/6 J mice were used for body weight study in Supplementary Fig. 7b. Lactate concentration in tumor interstitial fluid[2] Tumor interstitial fluid was collected from freshly resected MC38 tumor. Specimens were centrifuged against a 70 μm cell strainer at 4 °C for 5 min at 300 g. Flow-through tissue interstitial fluid was centrifuged at 4 °C for 5 min at 500 g. Supernatant were flash-frozen and stored at −80 °C before batch analysis. The lactate concentration was determined with lactate assay kit according to the manufacturer’s protocol. Lactate Concentration in plasma[2] Mouse blood (1 mL) was collected from lactate treated mice at different time points (0, 0.1, 0.5, 2, 10, and 24 h after lactate injection) using K3 EDTA blood collection tubes. Cells were removed from plasma by centrifugation for 10 mins at 2000 g at 4 oC. Lactate concentration in plasma was measured by BioProfile® FLEX2. Tumor free C57BL/6 J mice were used for this set of experiment. pH in blood[2] Mouse blood (1 mL) was collected from lactate treated mice at different time points (0, 0.1, 2, 10, and 24 h after lactate injection) to 1.5 mL Eppendorf tubes. The pH was measured immediately after collection with pH meter. Tumor free C57BL/6 J mice were used for this set of experiment. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
L-lactic acid occurs in small quantities in the blood and muscle fluid of humans and animals; the concentration of lactic acid in these fluids increases after vigorous activity. L-lactic acid is also present in the liver, kidneys, thymus gland, human amniotic fluid, and other organs and body fluids. A primed infusion study was performed /in humans/ using radioactive L-lactic acid. The virtual volume of distribution of lactate was 49.4% of body weight. The lactate pool size and turnover time were estimated as 0.029 g/kg and 18.4 min, respectively. In the body, lactate is distributed equivalently to, or slightly less than, total body water. It diffuses readily across cell membranes, primarily by passive transport; under certain conditions, the distribution could be uneven or the lactate pool could consist of several smaller pools with differing rate constants. The percutaneous absorption of topically applied 5% [14C]-lactic acid in an oil-in-water cream was measured using rats. After 3 days, 50% of the applied lactic acid had penetrated the skin. For more Absorption, Distribution and Excretion (Complete) data for LACTIC ACID (6 total), please visit the HSDB record page. Biodegradable nanoparticles (NP) of average size 75 nm and composed of poly(lactic acid, PLA) were prepared by single emulsion. Upon instillation into the vagina of mice in estrus, these particles undergo retrograde transport across the cervix to the uterus. Uterus lavage conducted after instillation of NP into the vagina indicated that proinflammatory signals such as RANTES and TNF were induced in the uterine environment, which is inimical to establishment of pregnancy. These NP are under investigation for contraceptive potential. Rhodamine B (RhB)-labeled PLA nanoparticles were prepared through surface grafting copolymerization of glycidyl methacrylate (GMA) onto PLA nanoparticles during the emulsion/evaporation process. RhB firstly interacts with sodium dodecyl sulfate (SDS) through electrostatic interaction to form hydrophobic complex (SDS-RhB). Due to the high-affinity of SDS-RhB with GMA, hydrophilic RhB can be successfully combined into PLA nanoparticles. The internalization of RhB-labeled PLA nanoparticles by macrophages was investigated with fluorescence microscope technology. The effects of the PLA nanoparticle surface nature and size on the internalization were investigated. The results indicate that the PLA particles smaller than 200 nm can avoid the uptake of phagocytosis. The bigger PLA particles (300 nm) with polyethylene glycol (PEG) surface showed less internalization by macrophage compared with those with poly(ethylene oxide-propylene oxide) copolymer (F127) or poly(vinyl alcohol) (PVA) surface. The "stealth" function of PEG on the PLA nanoparticles from internalization of macrophages due to the low protein adsorption is revealed by electrochemical impedance technology. Mucosal immunization is designed to induce strong immune responses at portal of pathogen entry. Unfortunately, mechanisms underlying the fate of the vaccine vector co-administered with antigens are still partially uncovered and limit further development of mucosal vaccines. Hence, poly(lactic acid) (PLA) nanoparticles being a versatile vaccine vehicle, we have analyzed the fate of these PLA nanoparticles during their uptake at intestinal mucosal sites, both in vivo and ex vivo, to decipher the mechanisms involved during this process. We first designed specific fluorescent PLA nanoparticles exhibiting strong colloidal stability after encapsulation of either 6-coumarin or CellTrace BODIPY before monitoring their transport through mucosa in the mouse ligated ileal loop model. The journey of the particles appears to follow a three-step process. Most particles are first entrapped in the mucus. Then, crossing of the epithelial barrier takes place exclusively through M-cells, leading to an accumulation in Peyer's patches (PP). Lastly, we noticed specific interaction of these PLA nanoparticles with underlying B cells and dendritic cells (DCs) of PP. Furthermore, we could document that DCs engulfing some nanoparticles could exhibit a TLR8+ specific expression. Specific targeting of these two cell types strongly supports the use of PLA nanoparticles as a vaccine delivery system for oral use. Indeed, following oral gavage of mice with PLA nanoparticles, we were able to observe the same biodistribution patterns, indicating that these nanoparticles specifically reach immune target required for oral immunization. Metabolism / Metabolites ... Propylene glycol ... is oxidized to lactic acid or pyruvic acid by two pathways. These two metabolites are then used by the body as sources of energy either by oxidation through the tricarboxylic acid cycle or by generation of glycogen through the glycolytic pathway. Lactic acid diffuses through muscle tissue and is transported to the liver in the bloodstream. In the liver, it is converted to glucose by gluconeogenesis. Lactic acid can also be further catabolized in the lactic acid cycle (also known as the Cori cycle). L-lactic acid is a normal metabolic intermediate produced by most mammalian cells and other organisms, such as bacteria; it is metabolized in preference to D-lactic acid in man, dogs, and rats. Lactic acid is converted to pyruvic acid by lactic acid dehydrogenase. In animals, lactate that is generated by anaerobic metabolism can be transported to other more aerobic tissues, such as the liver, where it can be reconverted to pyruvate. The pyruvate can then be further metabolized, reconverted to carbohydrate material as free glucose, or stored as glycogen. For more Metabolism/Metabolites (Complete) data for LACTIC ACID (8 total), please visit the HSDB record page. In August 2004, the US Food and Drug Administration approved a poly-L-lactic acid (PLLA)-based injectable medical device for restoration and/or correction of the signs of facial fat loss (lipoatrophy) in people with human immunodeficiency virus. As a result, the properties of the PLLA microparticles have received considerable interest from the medical community. Polylactides have a long-standing history of safe use in medical applications, such as pins, plates, screws, intra-bone and soft-tissue implants, and as vectors for sustained release of bioactive compounds. The L-isomer of polylactic acid is a biodegradable, biocompatible, biologically inert, synthetic polymer. Putatively, PLLA microparticles initiate neocollagenesis as a result of a normal foreign-body reaction to their presence. The build-up of collagen over time creates volume at the site of injection, while the PLLA microparticles are metabolized to carbon dioxide and water and expelled through the respiratory system. /Poly-L-lactic acid/ Polylactic acid (PLA) was introduced in 1966 for degradable surgical implants. Hydrolysis yields lactic acid, a normal intermediate of carbohydrate metabolism. Polyglycolic acid sutures have a predictable degradation rate which coincides with the healing sequence of natural tissues. Biological Half-Life The average effective half-life time of (32)P-chromic phosphate-poly L lactic acid ((32)P-CP-PLLA) was 11.8 days. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Lactic acid forms yellow to colorless crystals or syrupy 50% liquid. It has multiple uses in dyeing baths, as mordant in printing woolen goods, solvent for water-insoluble dyes. It is also used for reducing chromates in mordanting wool, in manufacture of cheese, confectionery. Lactic acid is a component of babies' milk formulas; acidulant in beverages; also used for acidulating worts in brewing. It is used in prepn of sodium lactate injections, and as ingredient of cosmetics, component of spermatocidal jellies. Other uses: for removing Clostridium butyricum in manufacture of yeast; dehairing, plumping, and decalcifying hides, solvent for cellulose formate, flux for soft solder. Lactic acid is used to manufacture lactates which are used in food products, in medicine, and as solvents. It is also a plasticizer, catalyst in the casting of phenolaldehyde resins. HUMAN EXPOSURE AND TOXICITY: Its effect on eye is similar to that of other acid of moderate strength, causing initial epithelial coagulation on cornea and conjunctiva, but having good prognosis if promptly washed off with water. In man, accidental intraduodenal administration of 100 mL 33% lactic acid was fatal within 12 hours. Hyperlactatemia and lactic acidosis are among the most dangerous and life-threatening side effect that occurs during therapy with some nucleoside reverse transcriptase inhibitors. Lactic acidosis is associated with both inherited and acquired metabolic diseases. Lactic acid metabolism in the presence of altered gluconeogenesis, anaerobic glycolysis, and acid-base balance is a major factor in many disorders. Lactic acid can be formed only from pyruvic acid; therefore, disorders that increase pyruvate concentration, enhance lactic acid formation, or reduce lactic acid degradation cause lactic acidosis. Inborn metabolic errors that are accompanied by derangement of metabolic pathways of glucose, pyruvate, amino acids, and organic acids as well as toxic and systemic conditions that promote tissue hypoxia or mitochondrial injury result in lactic acidosis. ANIMAL STUDIES: Applied to rabbit eyes in a standard manner, the reaction at twenty-four hours has been graded 8 on scale of 1 to 10. If allowed to remain on rabbit eyes, both the full strength acid and a 50% solution in water have caused corneal necrosis and persistent stromal scarring. Groups of male rats, five per group, were dosed with 0.5 mL of 130, 650, or 1300 mg/2000 kg body wt lactic acid via stomach tube; the control group received the same volume of water. Two rats of the 650-mg group and one rat of the 1300-mg group died within 24 hr of dosing. The rats were dosed with the same amounts of lactic acid after 8 days. Two rats of the 1300 mg group died; dyspnea, snivel, vomiting, and abdominal inflation were observed in these animals immediately after dosing. No overt toxic effects were observed in pigs given approximately 3.6-18 g/kg lactic acid in feed or water for up to 5 months. Drunken lamb syndrome has been described as lamb D-lactic acidosis syndrome. In developmental study, twelve mice were dosed daily with 570 mg/kg lactic acid by gavage on days 6 to 15 of gestation; a control group of 13 mice received distilled water. All dams were killed on day 18 of gestation. No significant difference was observed in gestational body weight gain between test and control animals, but feed consumption was significantly decreased as compared to control values. Also, relative maternal liver weight was significantly decreased as compared to controls. The only observed effect on the fetus was a statistically significant increase in delayed ossification of the parietal bones. Female rabbits were dosed orally with 0.1 - 0.2 g/kg lactic acid in 100 -150 mL water twice daily for 5 months, and five female rabbits were dosed orally with 0.1 - 0.7 g/kg lactic acid in 50 - 100 mL water twice daily for 16 months (13 months actual treatment). No tumors were reported after 5 or 16 months, respectively. Negative results were obtained when the mutagenic potential of lactic acid, 90.5% pure, in phosphate buffer was assayed in an Ames test using S. typhimurium strains TA92, TA1535, TA100, TA1537, TA94, and TA98 with metabolic activation. Negative results were obtained in an Ames test for 1000 ug/mL 11 mM lactic acid using a clonal subline of Chinese hamster fibroblasts derived from lung tissue in the absence of metabolic activation. Lactic acid was negative for chromosomal aberrations. ECOTOXICITY STUDIES: Feeding of 10% lactic acid to birds has been blamed for the development of polyneuritic crises resembling B1 deficiency on diets rich in carbohydrates, proteins or fats. IDENTIFICATION AND USE: Polylactic acid (PLA) is bioabsorbable polymer. It is used in the industrial packaging field or the biocompatible/bioabsorbable medical device market. PLA was first approved for soft tissue augmentation in Europe in 1999 for the cosmetic correction of scars and wrinkles. It is used in the US for restoration and/or correction of the signs of facial fat loss (lipoatrophy) in people with human immunodeficiency virus. HUMAN EXPOSURE AND TOXICITY: In August 2004, the US Food and Drug Administration approved a PLA-based injectable medical device for restoration and/or correction of the signs of facial fat loss (lipoatrophy) in people with human immunodeficiency virus. As a result, the properties of the PLA microparticles have received considerable interest from the medical community. Polylactides have a long-standing history of safe use in medical applications, such as pins, plates, screws, intra-bone and soft-tissue implants, and as vectors for sustained release of bioactive compounds. The L-isomer of polylactic acid is a biodegradable, biocompatible, biologically inert, synthetic polymer. Putatively, PLA microparticles initiate neocollagenesis as a result of a normal foreign-body reaction to their presence. The build-up of collagen over time creates volume at the site of injection, while the PLA microparticles are metabolized to carbon dioxide and water and expelled through the respiratory system. Injection procedure reactions to poly-L-lactic acid have been observed, consisting mainly of hematoma, bruising, edema, discomfort, inflammation, and erythema. The most common PLA adverse effect was the delayed occurrence of subcutaneous papules, which were confined to to the injection site and were typically palpable, asymptomatic, and nonvisible. Adverse reactions noted postmarketing: CNS- fatigue, lack of effectiveness, malaise; dermatologic - application-site discharge, ectropion, hypertrophy of skin, injection-site abscess, injection-site atrophy, injection-site fat atrophy, injection-site granuloma, injection-site reaction, skin rash, skin roughness, telangiectasias, visible nodules with or without inflammation or dyspigmentation; and miscellaneous- aching joints, allergic reaction, angioedema, brittle nails, colitis not otherwise specified, hair breakage, hypersensitivity reaction, photosensitivity reaction, quincke edema. ANIMAL STUDIES: In rat studies PLA was biocompatible and well tolerated by the tissues studied, and found to be negative for chromosomal mutagenicity. In vitro cell proliferation was studied on polylactides of varying molecular weights using rat epithelial cells under culture conditions. Overall, it was determined that satisfactory biocompatibility was exhibited, although some cell inhibition was also noted. In some early animal studies with PLA symptoms related to chronic inflammation (presence of macrophages, fibroblasts, giant cells and lymphocytes) were observed. These inflammatory changes were not related to bacterial infection. Toxicity Data LC50 (rat) = 7,940 mg/m3/4hr Interactions The effects of local myocardial administration of lactic acid and low-dose edaravone were investigated to determine if this combination provides benefits similar to mechanical postconditioning. We randomly divided 108 rats into 6 groups: sham, reperfusion injury, postconditioning (Post), lacticacid (Lac), low-dose edaravone (Eda), and lactic acid + low-dose edaravone (Lac+Eda). The left coronary arteries of the rats were occluded for 45 minutes, before the administration of the treatments. The rats were euthanized at different time points to examine the infarct size and serum markers of myocardial injury and apoptosis and measure the expression of signal pathway markers. We found that the infarct areas caused by ischemic-reperfusion injury were reduced largely by postconditioning and Lac+Eda injection; a similar trend was observed for serum markers of myocardial injury, apoptosis, and hemodynamic parameters. Compared with the Post group, the Lac+Eda group had similar blood pH values, levels of reactive oxygen species, mitochondrial absorbance, and levels of signal pathway marker. The Lac and Eda groups partly mimicked the protective role. These data suggest that local myocardial administration of lactic acid and low dose of edaravone initiates protective signal pathways of mechanical postconditioning and replicates the myocardial protection. Excretion of carbon dioxide and L-lactic acid through exhalation and perspiration provides olfactory signals to mosquitoes which allow them to find and bite humans; however, mosquito species differ in this regard. This study investigated upwind responses of Anopheles stephensi, mysorensis form, an important malaria vector in Asia, to carbon dioxide and L-lactic acid under laboratory conditions. While a minimal dose of carbon dioxide (90 ppm) activated the mosquitoes, 10 times this amount suppressed them. L-lactic acid alone did not produce a significant effect by itself, but addition of 6 ug/min of L-lactic acid to a range of 90 to 410 ppm carbon dioxide resulted in attraction. The results provide further support for the hypothesis that CO2 plays an important role in the host-seeking behavior of zoophilic mosquitoes, and suggests that L-lactic acid might play a more critical role than CO2 in the attraction of An. stephensi. During the pulmonary edema stage ... metabolic acidosis may occur because of increased lactic acid production in response to hypoxemia. /NO2-induced acute lung injury/ Burning and/or stinging is one of the most common concerns expressed by patients using topical therapies for treatment of dermatologic disorders. Topical lactic acid preparations often are used to treat dry scaly skin. In this study, we compared the level of burning/stinging reported by participants with application of lactic acid cream 10% containing strontium versus ammonium lactate lotion 12% and cetearyl alcohol lotion. The mean rating of burning/stinging reported for lactic acid cream 10% with strontium and cetearyl alcohol lotion was lower than ammonium lactate lotion 12% (P<.0001). Based on the study results, lactic acid cream 10% with strontium causes less burning/stinging than ammonium lactate lotion 12%. For more Interactions (Complete) data for LACTIC ACID (6 total), please visit the HSDB record page. A novel type of environmentally friendly packaging with antibacterial activity was developed from lauric arginate (LAE)-coating of polylactic acid (PLA) films after surface activation using a corona discharge. Scanning electron microscopy (SEM)-based analysis of the LAE/PLA films confirmed the successful coating of LAE on the PLA surface. The mechanical properties of the LAE/PLA films with different levels of LAE-coating (0% to 2.6%[w/w]) were essentially the same as those of the neat PLA film. The antibacterial activity of the LAE/PLA films against Listeria monocytogenes and Salmonella enterica Serovar typhimurium (S. typhimurium) was confirmed by a qualitative modified agar diffusion assay and quantitative JIS Z 2801:2000 method. Using the LAE/PLA film as a food-contact antimicrobial packaging for cooked cured ham, as a model system, suggested a potential application to inhibit L. monocytogenes and S. typhimurium on ham with a 0.07% (w/w) LAE coating on the PLA when high transparency is required, as evidenced from the 2 to 3 log CFU/tested film lower pathogen growth after 7 d storage but even greater antibacterial activity is obtained with a LAE coating level of 2.6% (w/w) but at the cost of a reduced transparency of the finished product. This article shows how we can simply develop functional green packaging of PLA for food with effective and efficient antimicrobial activity by use of LAE coating on the surface via corona discharge. PRACTICAL APPLICATION: The effectiveness of an innovative antimicrobial LAE-coated PLA film against foodborne pathogens was demonstrated. Importantly, the application of the LAE to form the LAE-coated PLA film can be customized within current film manufacturing lines Reversal of the visible signs of facial aging with the use of injectable products as an alternative to surgery has become more popular, with nearly 5 million procedures performed in the United States in 2012. Volume augmentation products, such as hyaluronic acid (HA), calcium hydroxylapatite (CaHA), and poly-L-lactic acid (PLLA), are often used in combination with one another and with neurotoxins for facial rejuvenation because of the complementary modes of action. This article presents 2 case reports involving patientspecific combinations of 2 different HA products, injectable PLLA, and CaHA with incobotulinumtoxinA or abobotulinumtoxinA. The combination of HA, CaHA, PLLA, and neurotoxins has resulted in outstanding outcomes for many patients, with no clinical evidence of increased adverse events secondary to combination therapy. /Poly-L-lactic acid/ Degradable polymer-based materials are attractive in orthopedics and dentistry as an alternative to metallic implants for use as bone fixatives. Herein, a degradable polymer poly(lactic acid) (PLA) was combined with novel hybrid nanopowder of carbon nanotubes (CNTs)-calcium phosphate (CP) for this application. In particular, CNTs-CP hybrid nanopowders (0.1 and 0.25% CNTs) were prepared from the solution of ionically modified CNTs (mCNTs), which was specifically synthesized to be well-dispersed and thus to effectively adsorb onto the CP nanoparticles. The mCNTs-CP hybrid nanopowders were then mixed with PLA (up to 50%) to produce mCNTs-CP-PLA nanocomposites. The mechanical tensile strength of the nanocomposites was significantly improved by the addition of mCNTs-CP hybrid nanopowders. Moreover, nanocomposites containing low concentration of mCNTs (0.1%) showed significantly stimulated biological responses including cell proliferation and osteoblastic differentiation in terms of gene and protein expressions. Based on this study, the addition of novel mCNT-CP hybrid nanopowders to PLA biopolymer may be considered a new material choice for developing hard tissue implants. A temporary cardiovascular stent device by bioabsorbable materials might reduce late stent thrombosis. A water-soluble amphiphilic phospholipid polymer bearing phosphorylcholine groups (PMB30W) was blended with a high-molecular-weight poly(l-lactic acid) (PLLA) to reduce unfavorable tissue responses at the surface. The PLLA implants and the polymer blend (PLLA/PMB30W) implants were inserted into subcutaneous tissues of rats, the infrarenal aorta of rats, and the internal carotid arteries of rabbits. After 6 months subcutaneous implantation, the PLLA/PMB30W maintained high density of phosphorylcholine groups on the surface without a significant bioabsorption. After intravascular implantation, the cross-sectional areas of polymer tubing with diameters less than 1.6 mm were histomorphometrically measured. Compared to the PLLA tubing, the PLLA/PMB30W tubing significantly reduced the thrombus formation during 30 d of implantation. Human peripheral blood mononuclear cells were cultured on the PLLA and the PLLA/PMB30W to compare inflammatory reactions. Enzyme-linked immunosorbent assay quantified substantially decreased proinflammatory cytokines in the case of the PLLA/PMB30W. They were almost the same level as the negative controls. Thus, we conclude that the phosphorylcholine groups could reduce tissue responses significantly both in vivo and in vitro, and the PLLA/PMB30W is a promising material for preparing temporary cardiovascular stent devices. Non-Human Toxicity Values LD50 Rat oral 3730 mg/kg LD50 Guinea pigs oral 1810 mg/kg LD50 Mouse sc 4500 mg/kg LD50 Mouse oral 4875 mg/kg LC50 Rat inhalation 7.94 mg/L/4 hr |
| 参考文献 | |
| 其他信息 |
Lactic acid appears as a colorless to yellow odorless syrupy liquid. Corrosive to metals and tissue. Used to make cultured dairy products, as a food preservative, and to make chemicals.
2-hydroxypropanoic acid is a 2-hydroxy monocarboxylic acid that is propanoic acid in which one of the alpha-hydrogens is replaced by a hydroxy group. It has a role as a Daphnia magna metabolite and an algal metabolite. It is functionally related to a propionic acid. It is a conjugate acid of a lactate. A normal intermediate in the fermentation (oxidation, metabolism) of sugar. The concentrated form is used internally to prevent gastrointestinal fermentation. (From Stedman, 26th ed) Sodium lactate is the sodium salt of lactic acid, and has a mild saline taste. It is produced by fermentation of a sugar source, such as corn or beets, and then, by neutralizing the resulting lactic acid to create a compound having the formula NaC3H5O3. Lactic acid was one of active ingredients in Phexxi, a non-hormonal contraceptive agent that was approved by the FDA on May 2020. Lactic Acid has been reported in Drosophila melanogaster, Sambucus ebulus, and other organisms with data available. Lactic Acid, DL- is the racemic isomer of lactic acid, the biologically active isoform in humans. Lactic acid or lactate is produced during fermentation from pyruvate by lactate dehydrogenase. This reaction, in addition to producing lactic acid, also produces nicotinamide adenine dinucleotide (NAD) that is then used in glycolysis to produce energy source adenosine triphosphate (ATP). A normal intermediate in the fermentation (oxidation, metabolism) of sugar. The concentrated form is used internally to prevent gastrointestinal fermentation. (From Stedman, 26th ed) Drug Indication For use as an alkalinizing agent. Mechanism of Action Lactate ions are metabolized ultimately to carbon dioxide and water, which requires the consumption of hydrogen cations. Therapeutic Uses /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Lactic acid is included in the database. (VET): Has been used as a caustic, and in dilute solutions to irrigate tissues; as an intestinal antiseptic and antiferment. A 10% solution is used as a bactericidal agent on the skin of neonates. ... A 16.7% solution in flexible collodion is used to remove warts and small cutaneous tumors. Acidulant Delayed-Action Preparations; Membranes, Artificial; Dental Implants; Drug Delivery Systems For restoration and/or correction of the signs of facial fat loss (lipoatrophy) in people with human immunodeficiency virus. EXPL THER We report the use of injectable poly-L-lactic acid (PLLA) for volume restoration in a 45-year-old white female who was concerned about the appearance of her hands. The patient expressed a desire for long-term restoration, and selected injectable PLLA because of its known 2-year duration of effect, although she was informed that injectable PLLA is not FDA-approved for use in the hands. After reconstitution with 8 mL of diluent plus lidocaine, 0.1-0.2-mL aliquots of injectable PLLA were injected into selected sites, up to 5 mL per hand. The patient underwent three identical treatments, followed by postinjection use of moisturizing cream and massage; improvement in appearance was noted by the patient between the second and third treatments. Correction was maintained for at least 18 months, with no adverse events. We have also briefly reviewed the literature on the use of injectable PLLA for volume restoration in the hand. /Poly-L-lactic acid/ Characteristics of the aging face include soft tissue atrophy, loss of skin elasticity resulting in excess facial skin, and gravitational descent or ptosis of facial soft tissues. Poly-L-lactic acid (PLLA) is a synthetic biodegradable polymer that provides soft tissue augmentation through stimulation of an inflammatory tissue response with subsequent collagen deposition. The /paper/ discuss the special considerations inherent in facial aging, describe the mechanism of action and indications for a new PLLA filler under consideration for Food and Drug Administration (FDA) approval ... and detail the results of a two-year off-label pilot study with the product. 106 patients /were treated/ with PLLA in an off-label indication, as part of a pilot study while /the product/ was being evaluated for FDA approval for cosmetic indications. All patients were followed up for two years to help develop a protocol for injection technique. The age range of patients in this series was 40 to 78 years. Three patients were male and 103 were female. Patients received an average injection of 1.6 vials per session, over an average of 2.3 sessions, to achieve volume restoration in the tear trough, midface, malar region, nasolabial folds, prejowl area, mandibular border, and mandibular angle. The /study/ achieved 100% follow-up with 99.1% patient satisfaction. The rate of nodule formation was 4.7% at a minimum follow-up of two years. Because of its unique mechanism of action, PLLA for nonsurgical facial rejuvenation requires meticulous injection technique with special considerations for optimizing outcomes and minimizing adverse events. /Poly-L-lactic acid/ For more Therapeutic Uses (Complete) data for Polylactic acid (17 total), please visit the HSDB record page. Drug Warnings Defer use of poly-L-lactic acid in any person with active skin inflammation or infection in or near the treatment area until the inflammatory or infectious process has been controlled. /Poly-L-lactic acid/ Use poly-L-lactic acid in the deep dermis or subcutaneous layer. Avoid superficial injections. Take special care when using poly-L-lactic acid in area of thin skin. /Poly-L-lactic acid/ Safety and effectiveness of treatment in the periorbital area have not been established. Do not overcorrect (overfill) a contour deficiency because the depression should gradually improve within several weeks as the treatment effect of poly-L-lactic acid occurs. /Poly-L-lactic acid/ For more Drug Warnings (Complete) data for Polylactic acid (16 total), please visit the HSDB record page. Pharmacodynamics Lactic acid produces a metabolic alkalinizing effect. |
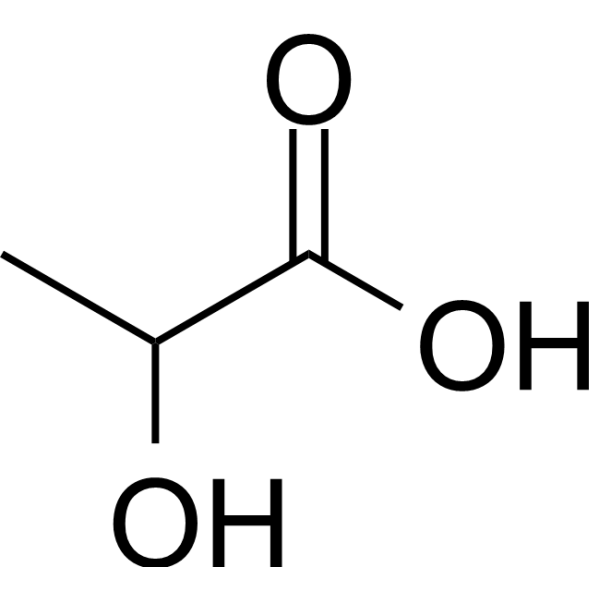
| 分子式 |
C3H6O3
|
|---|---|
| 分子量 |
90.0779
|
| 精确质量 |
90.031
|
| CAS号 |
50-21-5
|
| 相关CAS号 |
Lactate calcium;814-80-2;Lactate sodium;72-17-3;Lactate potassium;996-31-6
|
| PubChem CID |
612
|
| 外观&性状 |
Colorless to light yellow liquid
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
227.6±0.0 °C at 760 mmHg
|
| 熔点 |
18ºC
|
| 闪点 |
109.9±16.3 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.451
|
| LogP |
-0.7
|
| tPSA |
57.53
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
6
|
| 分子复杂度/Complexity |
59.1
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O([H])C([H])(C(=O)O[H])C([H])([H])[H]
|
| InChi Key |
JVTAAEKCZFNVCJ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C3H6O3/c1-2(4)3(5)6/h2,4H,1H3,(H,5,6)
|
| 化学名 |
2-hydroxypropanoic acid
|
| 别名 |
lactic acid; 2-hydroxypropanoic acid; DL-Lactic acid; 50-21-5; 2-hydroxypropionic acid; lactate; Milk acid; Tonsillosan
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~100 mg/mL (~1110.12 mM)
DMSO : ~100 mg/mL (~1110.12 mM) |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 11.1012 mL | 55.5062 mL | 111.0124 mL | |
| 5 mM | 2.2202 mL | 11.1012 mL | 22.2025 mL | |
| 10 mM | 1.1101 mL | 5.5506 mL | 11.1012 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。