| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 靶点 |
Folate-dependent enzymes (thymidylate synthase, dihydrofolate reductase cofactor) [1][2]
|
|---|---|
| 体外研究 (In Vitro) |
当单独使用 MTX 时,异常细胞 (Abs) 和微核双核细胞 (MNBN) 的百分比呈浓度依赖性增加。核分裂指数 (NDI) 随着 MTX 浓度的升高而下降。同样,在检测的每个 MTX 浓度下,有丝分裂指数 (MI) 也同样下降。添加 50 μg/mL 的亚叶酸可显着降低 MNBN (40-68%) 和 Abs (36-77%) 的百分比。此外,在 5 μg/mL 亚叶酸下,观察到抑制作用(MNBN 为 12% 至 54%,Abs 为 20% 至 61%)[1]。
亚叶酸钙(Leucovorin Calcium)以剂量依赖性方式保护V79细胞免受甲氨蝶呤诱导的染色体损伤。甲氨蝶呤(1 μM)单独处理使染色体畸变率从对照组的2.3%升高至18.6%;预处理、同时处理或后处理亚叶酸钙(Leucovorin Calcium)(0.1 μM、1 μM、10 μM)可降低畸变率:0.1 μM时降至10.2%,1 μM时降至5.8%,10 μM时降至3.1%(接近对照组水平)。其机制为提供活性四氢叶酸,弥补甲氨蝶呤(二氢叶酸还原酶抑制剂)导致的叶酸缺乏,减少DNA合成障碍和染色体断裂[1] |
| 体内研究 (In Vivo) |
接受甲氨蝶呤 (MTX) 三周后,用甲酰四氢叶酸(7.0 mg/kg;腹腔注射;每隔一天;对于 Balb/c 幼年雄性小鼠)治疗似乎可以抵消这种生长抑制(长期给药时,MTX 会减少小鼠的骨骼生长) [2]。
亚叶酸钙(Leucovorin Calcium)可部分逆转甲氨蝶呤诱导的幼龄小鼠骨骼生长抑制。腹腔注射甲氨蝶呤(20 mg/kg,每周1次,持续4周)使小鼠胫骨长度较对照组减少18%,生长板厚度减少25%;同时腹腔注射亚叶酸钙(Leucovorin Calcium)(5 mg/kg,每周1次)可缓解该效应:胫骨长度仅减少7%,生长板厚度减少10%;剂量增至10 mg/kg时,胫骨长度和生长板厚度分别减少4%和6%,接近对照组水平。它通过补充活性叶酸,维持骨骼细胞增殖和分化所需的叶酸依赖性代谢过程,减轻甲氨蝶呤对生长板软骨细胞的毒性[2] |
| 细胞实验 |
V79细胞染色体畸变实验:V79细胞以5×10³个/孔接种于6孔板,培养24小时后分为三组:对照组、甲氨蝶呤组(1 μM)、甲氨蝶呤+亚叶酸钙(Leucovorin Calcium)组(0.1 μM、1 μM、10 μM,分别于甲氨蝶呤处理前1小时、同时或处理后1小时加入)。继续培养48小时后,加入秋水仙素处理2小时,收集细胞经低渗处理、固定、制片和Giemsa染色,显微镜下计数每100个中期分裂相细胞的染色体畸变率(包括断裂、缺失、易位等)[1]
|
| 动物实验 |
Animal/Disease Models: 24 Balb/c young growing male mice aged 3 weeks (11.88 ± 0.25 g)[2]
Doses: 7.0 mg/kg Route of Administration: intraperitoneal (ip)injection; every second day; for 3 weeks Experimental Results: Following methotrexate (MTX) administration appears to reverse this growth inhibition. Skeletal growth assay in mice: 3-week-old female mice were randomly divided into three groups (10 mice per group): control group, methotrexate group, and methotrexate + Leucovorin Calcium groups (5 mg/kg and 10 mg/kg subgroups). Methotrexate was administered via intraperitoneal injection at 20 mg/kg once weekly for 4 weeks; Leucovorin Calcium was administered via intraperitoneal injection at the corresponding doses, consistent with the methotrexate administration schedule. At the end of the experiment, mice were sacrificed by cervical dislocation. Bilateral tibias were isolated to measure tibial length; proximal tibial growth plate tissues were collected, fixed, decalcified, embedded, sectioned, and stained with HE. Growth plate thickness was measured under a microscope and chondrocyte morphology was observed [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration, leucovorin is rapidly absorbed. The apparent bioavailability of leucovorin was 97% for 25 mg, 75% for 50 mg, and 37% for 100 mg. Duration of action: All routes: 3 to 6 hours. Onset of action: Oral: 20 to 30 minutes. Intramuscular: 10 to 20 minutes. Intravenous: Less than 5 minutes. Crosses blood-brain barrier in moderate amounts; largely concentrated in liver. Elimination: Renal: 80-90%. Fecal: 5-8%. For more Absorption, Distribution and Excretion (Complete) data for LEUCOVORIN (10 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic and intestinal mucosal, the main metabolite being the active 5-methyltetrahydrofolate. Leucovorin is readily converted to another reduced folate, 5,10-methylenetetrahydrofolate, which acts to stabilize the binding of fluorodeoxyridylic acid to thymidylate synthase and thereby enhances the inhibition of this enzyme. In vivo, leucovorin calcium is rapidly and extensively converted to other tetrahydrofolic acid derivatives including 5-methyl tetrahydrofolate, which is the major transport and storage form of folate in the body. /Leucovorin calcium/ Hepatic and intestinal mucosal, mainly to 5-methyltetrahydrofolate (active). After oral administration, leucovorin is substantially (greater than 90%) and rapidly (within 30 minutes) metabolized. Metabolism is less extensive (about 66% after intravenous and 72% after intramuscular administration) and slower with parenteral administration. Biological Half-Life 6.2 hours Terminal half-life for total reduced folates: 6.2 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Leucovorin (folinic acid; 5-formyltetrahydrofolic acid) and its levo- isomer, levoleucovorin, are folic acid derivatives that are normal components of breastmilk. Because leucovorin and levoleucovorin are used as therapeutic agents with potentially toxic drugs such as fluorouracil or methotrexate, the LactMed record of the drug it is used with should be consulted. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding ~15% Toxicity Data Mouse(iv): LD50 732 mg/kg Interactions Concurrent use of leucovorin /with fluorouracil/ may increase the therapeutic and toxic effects of fluorouracil; although the two medications may be used together for therapeutic advantage, caution is necessary. Exposure of tumor cells to reduced folates /like leucovorin/ before or with the fluoropyrimidines, 5-fluorouracil or 5-fluoro-2'deoxyuridine, results in a substantial increase in the activity of these drugs. Available evidence suggests that the mechanism of this synergism is a kinetic stabilization of complex formed between thymidylate synthase and fluorodeoxyuridylate that also involves a mole of the cofactor for the thymidylate synthase reaction, 5,10-methylenetetrahydrofolate. This effect results in an extended time of depletion of thymidine nucleotides with a resultant increased level of cell death. Large dose of leucovorin may counteract the anticonvulsant effects of these medications: /barbiturate anticonvulsants, hydantoin anticonvulsants, primidone/. The Sprague Dawley rat was used to demonstrate the effect of nitrous oxide, with and without folinic pretreatment, on reproductive indices and fetal development. ... Groups of animals were exposed to 70-75% nitrous oxide on day 9 of pregnancy with or without folinic acid 0.1 mg ip 12 hr before, and immediately before, exposure. Subsequent fetal development was compared with that of various control groups. There were no significant differences in fetal survival, but fetal wt were reduced in both groups exposed to nitrous oxide. Of the indices of skeletal maturity, the number of ossified sternebrae was reduced only in the nitrous oxide group not receiving folinic acid. The incidence of major skeletal abnormalities in the untreated nitrous oxide group was significantly increased to five times that of the control groups, whereas the incidence in the nitrous oxide group receiving folinic acid was not significantly different from control. It is concluded that pretreatment with folinic acid can at least partially reduce the teratogenic effects of nitrous oxide in the rat. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Leucovorin is indicated for use in combination with agents such as fluorouracil or high-dose methotrexate, as second-line treatment of squamous cell head and neck carcinoma. /NOT included in US product labeling/ Antidotes Leucovorin is indicated as a antidote to the toxic effects of folic acid antagonists such as methotrexate, pyrimethamine, or trimethoprim. Leucovorin also is indicated as a rescue after high-dose methotrexate therapy in osteosarcoma and as a part of chemotherapeutic treatment programs in the management of several forms of cancer. /Included in US product labeling/ Leucovorin is indicated to treat megaloblastic anemias associated with sprue, nutritional deficiency, pregnancy, and infancy when oral folic acid therapy is not feasible. Leucovorin is not recommended for use in the treatment of pernicious anemia or other megaloblastic anemias secondary to lack of vitamin B12, since it may produce a hematologic remission while neurologic manifestations continue to progress. /Included in US product labeling/ For more Therapeutic Uses (Complete) data for LEUCOVORIN (7 total), please visit the HSDB record page. Drug Warnings Since allergic reactions have been reported following oral and parenteral administration of folic acid, the possibility of allergic reactions to leucovorin should be considered. There is a potential danger in administering leucovorin to patients with undiagnosed anemia, as leucovorin may obscure the diagnosis of pernicious anemia by alleviating hematologic manifestations of the disease while allowing neurologic complications to progress. This may result in severe nervous system damage before the correct diagnosis is made. Adequate doses of Vitamin Bl2 may prevent, halt, or improve neurologic changes caused by pernicious anemia. When leucovorin rescue is used in conjunction with high dose methotrexate therapy, the drugs should be administered only by physicians experienced in cancer chemotherapy, in centers where facilities for measuring blood methotrexate concentrations are available. Leucovorin is usually effective in counteracting severe methotrexate toxicity in these regimens, but toxic reactions to methotrexate may occur despite leucovorin therapy, especially when the half-life of methotrexate is increased (eg, renal dysfunction). Therefore, it is extremely important that leucovorin be administered until the blood concentration of methotrexate declines to nontoxic concentrations. Since leucovorin calcium enhances the toxicity of fluorouracil, adjunctive therapy with leucovorin calcium and fluorouracil should be given only by, or under the supervision of, physicians experienced in cancer chemotherapy and in the use of antimetabolites. /Leucovorin calcium/ For more Drug Warnings (Complete) data for LEUCOVORIN (10 total), please visit the HSDB record page. Pharmacodynamics Leucovorin is one of several active, chemically reduced derivatives of folic acid. It is useful as an antidote to drugs which act as folic acid antagonists. Leucovorin is a mixture of the diastereoisomers of the 5-formyl derivative of tetrahydrofolic acid (THF). The biologically active compound of the mixture is the (-)-l-isomer, known as Citrovorum factor or (-)-folinic acid. Leucovorin does not require reduction by the enzyme dihydrofolate reductase in order to participate in reactions utilizing folates as a source of “one-carbon” moieties. Administration of leucovorin can counteract the therapeutic and toxic effects of folic acid antagonists such as methotrexate, which act by inhibiting dihydrofolate reductase. Leucovorin has also been used to enhance the activity of fluorouracil. Leucovorin Calcium is the active metabolite of folic acid (calcium 5-formyltetrahydrofolate), which can directly act as a coenzyme for folate-dependent enzymes involved in DNA, RNA, and protein synthesis [1][2] - Its core mechanism is to supplement folate deficiency induced by methotrexate (a dihydrofolate reductase inhibitor), reversing methotrexate-induced toxicity to rapidly proliferating cells (e.g., bone marrow cells, gastrointestinal mucosal cells, tumor cells) [1] - Literature [1] confirms its in vitro protective effect against methotrexate-induced chromosomal damage, reflecting genotoxicity protection; Literature [2] shows its in vivo ability to partially reverse methotrexate-induced inhibition of skeletal growth in young mice, protecting growth plate chondrocyte function [1][2] |
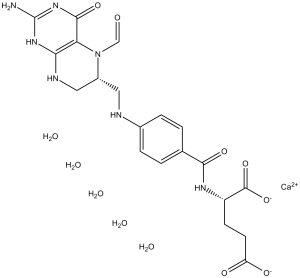
| 分子式 |
C21H25CAN7O7.5H2O
|
|
|---|---|---|
| 分子量 |
601.58
|
|
| 精确质量 |
511.112
|
|
| CAS号 |
1492-18-8
|
|
| 相关CAS号 |
Folinic acid;58-05-9;Folinic acid calcium salt pentahydrate;6035-45-6;Folinic acid calcium hydrate;1097832-14-8
|
|
| PubChem CID |
135403648
|
|
| 外观&性状 |
Off-white to light yellow solid powder
|
|
| 熔点 |
240-250ºC
|
|
| LogP |
1.411
|
|
| tPSA |
198.1
|
|
| 氢键供体(HBD)数目 |
7
|
|
| 氢键受体(HBA)数目 |
10
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
34
|
|
| 分子复杂度/Complexity |
911
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
C1C(N(C2=C(N1)N=C(NC2=O)N)C=O)CNC3=CC=C(C=C3)C(=O)N[C@@H](CCC(=O)O)C(=O)O
|
|
| InChi Key |
NPPBLUASYYNAIG-ZIGBGYJWSA-L
|
|
| InChi Code |
InChI=1S/C20H23N7O7.Ca.5H2O/c21-20-25-16-15(18(32)26-20)27(9-28)12(8-23-16)7-22-11-3-1-10(2-4-11)17(31)24-13(19(33)34)5-6-14(29)30;;;;;;/h1-4,9,12-13,22H,5-8H2,(H,24,31)(H,29,30)(H,33,34)(H4,21,23,25,26,32);;5*1H2/q;+2;;;;;/p-2/t12?,13-;;;;;;/m0....../s1
|
|
| 化学名 |
L-Glutamic acid, N-(4-(((2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl)amino)benzoyl)-, calcium salt (1:1), pentahydrate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 (2). 该产品在溶液状态不稳定,请现配现用。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6623 mL | 8.3114 mL | 16.6229 mL | |
| 5 mM | 0.3325 mL | 1.6623 mL | 3.3246 mL | |
| 10 mM | 0.1662 mL | 0.8311 mL | 1.6623 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Study of Pembrolizumab (MK-3475) Versus Chemotherapy in Chinese Participants With Stage IV Colorectal Cancer (MK-3475-C66)
CTID: NCT05239741
Phase: Phase 3 Status: Recruiting
Date: 2024-12-02