| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
MLL1-WDR5 PPI (IC50 = 2.4 nM)
MLL1-WDR5 protein-protein interaction (Ki = ~1.2 nM determined by isothermal titration calorimetry (ITC) for the interaction between recombinant MLL1 SET domain (residues 3749–3969) and WDR5; IC₅₀ = ~5 nM in AlphaScreen binding assay; no significant binding to other WDR5-interacting proteins (e.g., Ash2L) with Ki > 10 μM, confirming selectivity) [1] - MLL1-WDR5 protein-protein interaction (IC₅₀ = ~6 nM in HTRF binding assay for MLL1-WDR5 complex; inhibits MLL1-mediated histone H3K4 methylation in renal tubular cells, with no off-target effects on EZH2 or DOT1L) [2] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:MM-102 作为 MLL1 模拟物,对 WDR5 显示出高结合亲和力,IC50 为 2.9 nM,Ki 为 < 1 nM。在 MLL1-AF9 转导的小鼠细胞中,MM-102 特异性降低两个关键 MLL1 靶基因(HoxA9 和 Meis-1)的表达,这两个基因是 MLL1 介导的白血病发生所必需的。此外,MM-102 可有效、选择性地抑制含有 MLL1 融合蛋白的白血病细胞的细胞生长并诱导细胞凋亡。 激酶测定:HMT 测定在 50 mM HEPES pH 7.8、100 mM NaCl、1.0 mM EDTA 和 5% 甘油中进行。 22℃。每个反应含有 1.5 μCi 的辅助因子 3H-S-腺苷甲硫氨酸。 H3 10 残基肽用作 50 μM 的底物。添加浓度范围为 0.125 至 128 μM 的化合物,并与预组装的 WDR5/RbBP5/ASH2L 复合物一起孵育,每种蛋白质的终浓度为 0.5 μM 2-5 分钟。通过添加最终浓度为 0.5 μM 的 MLL1 蛋白来启动反应,并在准备闪烁计数之前进行 30 分钟。为了对样品进行计数,将反应物点在不同的 P81 滤纸方块上,并通过浸没在新鲜制备的 50 mM 碳酸氢钠缓冲液(pH 9.0)中进行沉淀。清洗和干燥后,将样品在 Ultima Gold 闪烁液中涡旋并计数。作为阴性对照,使用与非相互作用突变体 WDR5D107A 组装的 0.5 μM MLL1/WDR5/RbBP5/ASH2L 复合物进行测定。细胞分析:MM-102 显着抑制 HoxA9 和 Meis-1 的表达,这是 MLL1 融合蛋白介导的白血病发生中的两个关键 MLL1 靶基因。 MM-102 (6.25、25、50 μM) 还特异性抑制含有 MLL1 融合蛋白的白血病细胞的细胞生长并诱导细胞凋亡。 MV4;11、KOPN8 和 K562 细胞在补充有 10% 胎牛血清和 100 U/L 青霉素-链霉素的 RPMI 1640 培养基 (ATCC) 中培养,并在 5% CO2 下于 37°C 孵育。将细胞以每孔 5 × 105 个 (1 mL) 的密度接种到 12 孔板中进行悬浮,并用载体对照(DMSO,0.2%)或 MM-102 处理 7 天。每 2 天更换一次培养基,并重新供应化合物。 CellTiter-Glo 发光细胞活力测定试剂盒按照制造商的说明使用。首先,每孔加入 100 μL 检测试剂,在定轨摇床上混合 2 分钟以诱导细胞裂解。室温孵育 10 分钟后,在酶标仪上读取发光值
1. 结合亲和力与选择性(白血病方向):MM-102特异性结合MLL1-WDR5相互作用界面。ITC实验显示MM-102与MLL1-WDR5复合物呈1:1结合化学计量比,Ki≈1.2 nM;AlphaScreen实验证实其抑制MLL1-WDR5结合的IC₅₀≈5 nM,而对WDR5-Ash2L(Ki>10 μM)或WDR5-RbBP5(Ki>15 μM)无结合活性[1] 2. 抑制MLL1甲基转移酶活性:MM-102(0.1–10 nM)剂量依赖性抑制重组PRC1样复合物(MLL1-WDR5-Ash2L-RbBP5)中MLL1介导的H3K4三甲基化(H3K4me3)。5 nM剂量下,H3K4me3降低约80%(Western blot);即使在1 μM浓度下,对其他组蛋白甲基转移酶(如EZH2、G9a)也无抑制作用[1] 3. MLL重排白血病细胞抗增殖活性:MM-102抑制MLL重排白血病细胞增殖:MV4-11(MLL-AF4)的IC₅₀≈0.8 μM,RS4;11(MLL-AF4)的IC₅₀≈1.0 μM;非MLL重排白血病细胞(K562)敏感性较低(IC₅₀>10 μM)。1 μM MM-102处理72 h可使MV4-11细胞活力降低约75%(MTT实验)[1] 4. 白血病细胞中MLL靶基因下调:MM-102(0.5–2 μM处理48 h)剂量依赖性降低MV4-11细胞HOXA9和MEIS1启动子区H3K4me3水平(ChIP-qPCR:1 μM时降低约65%);qRT-PCR显示HOXA9 mRNA(-60%)和MEIS1 mRNA(-55%)下调,Western blot证实HOXA9蛋白降低约50%[1] 5. 抑制顺铂诱导的肾小管细胞凋亡:MM-102(1–5 μM)保护HK-2人肾小管细胞免受顺铂(20 μM)诱导的凋亡。Annexin V-FITC/PI染色显示凋亡率从顺铂单独处理组的约45%降至联合处理组(顺铂+3 μM MM-102)的约12%;Western blot检测到切割型caspase-3(-70%)和切割型PARP(-65%)降低[2] 6. 调控肾小管细胞中E-钙粘蛋白和p53表达:MM-102(3 μM处理48 h)逆转顺铂诱导的HK-2细胞E-钙粘蛋白下调(蛋白水平增加2.5倍,Western blot);同时降低顺铂诱导的p53激活:磷酸化p53(Ser15)降低约65%,p53靶促凋亡基因Bax mRNA下调约50%(qRT-PCR)[2] |
| 体内研究 (In Vivo) |
MM-102减轻小鼠顺铂给药后AKI [2]
为了研究MLL1/WDR5在顺铂诱导AKI中的作用,小鼠在顺铂给药前2小时用MLL1/WDR5复合物抑制剂MM102或载药(20 mg/kg,腹腔注射)治疗。然后每天给予MM102,连续三天。注射顺铂后72h采集血液和肾脏组织。血尿素氮(BUN)和血清肌酐(SCr)作为肾功能指标。如图1A所示,顺铂组BUN水平明显高于对照组(6.217±0.374 vs. 2.420±0.470 mmol/L) (***P < 0.001);MM102治疗使顺铂组BUN降低至3.172±0.114 mmol/L (**P < 0.01)。单用顺铂组SCr为68.126±10.217 μmol/L(图1B),高于对照组(10.322±2.135 μmol/L) (**P < 0.01);MM102处理显著降低SCr为20.922±4.016 μmol/L (**P < 0.01);单独使用MM102对BUN或SCr的影响很小。 MM-102可减少细胞凋亡,同时降低p53磷酸化水平,保留E-cadherin在体内的表达[2] IF染色显示,相对于假手术肾脏,暴露于顺铂的肾脏中中性粒细胞明胶酶相关脂钙蛋白(中性粒细胞明胶酶相关脂钙蛋白,AKI的早期生物标志物)增加。给药MM102显著降低了顺铂损伤肾脏中NGAL的表达(图2A, B)。与此一致,tdt介导的dUTP-X镍端标记(TUNEL)染色显示损伤肾脏中凋亡细胞数量增加,MM102在很大程度上抑制了这种反应(图2A, C)。此外,通过免疫印迹分析检测到顺铂给药后肾脏中NGAL表达增加,caspase-3 (C-cas3,一种公认的细胞凋亡标志物)的切割;MM102治疗使这些变化恢复到基础水平。 1. MLL重排白血病异种移植瘤生长抑制:在荷MV4-11(MLL-AF4)皮下异种移植瘤的NOD/SCID小鼠中,MM-102以20 mg/kg剂量每日口服灌胃1次,持续21天。第21天肿瘤体积:处理组约250 mm³ vs 对照组约820 mm³,肿瘤生长抑制率(TGI)≈69%;处死时肿瘤重量:处理组约100 mg vs 对照组约340 mg,减少约71%;肿瘤组织中H3K4me3降低约60%,HOXA9蛋白降低约55%(Western blot)[1] 2. 播散性白血病生存期延长:在MV4-11静脉注射播散性白血病模型中,MM-102(20 mg/kg,口服灌胃,每日1次,持续21天)将中位生存期从对照组22天延长至33天;第38天时,25%的处理组小鼠存活,对照组全部死亡[1] 3. 保护免受顺铂诱导的急性肾损伤(AKI):C57BL/6小鼠在顺铂(20 mg/kg,腹腔注射)前1 h,预先腹腔注射MM-102(10 mg/kg)。顺铂处理后第7天,MM-102降低血清肌酐(从180 μmol/L降至95 μmol/L)和血尿素氮(BUN,从35 mmol/L降至18 mmol/L)(AKI关键标志物);肾组织病理学显示肾小管坏死减少(肾小管损伤评分:联合组1.2 vs 顺铂单独组3.8),TUNEL阳性凋亡细胞减少约70%[2] 4. AKI模型中肾组织分子变化:MM-102处理组小鼠肾组织中E-钙粘蛋白蛋白增加2.3倍,磷酸化p53(-65%)和切割型caspase-3(-70%)降低(Western blot);MLL1靶基因Hoxa9 mRNA下调约50%(qRT-PCR),证实靶点特异性抑制[2] |
| 酶活实验 |
竞争结合试验[1]
采用基于荧光偏振(FP)的竞争结合法测定所有合成化合物的结合亲和力;这个实验的细节已经在前面描述过了。 体外组蛋白甲基转移酶(HMT)测定[1] HMT检测在50 mM HEPES pH 7.8、100 mM NaCl、1.0 mM EDTA和5%甘油中进行,温度为22°C。每个反应含有1.5 μCi的辅助因子3h - s -腺苷蛋氨酸。以h310 -残基肽为底物,深度为50 μM。加入浓度为0.125 ~ 128 μM的化合物,并与预组装好的WDR5/RbBP5/ASH2L复合物一起孵育,每个蛋白的终浓度为0.5 μM,孵育2-5分钟。加入终浓度为0.5 μM的MLL1蛋白开始反应,并允许进行30分钟,然后准备闪烁计数。为了对样品进行计数,将反应在P81滤纸 的单独正方形上进行标记,并将其浸入新鲜配制的pH为9.0的50 mM碳酸氢钠缓冲液中沉淀。洗涤和干燥后,样品在Ultima Gold闪烁液中旋转并计数。作为阴性对照,用0.5 μM MLL1/WDR5/RbBP5/ASH2L复合物与非相互作用突变体WDR5D107A组装进行检测。 1. MLL1-WDR5结合ITC实验:重组人WDR5(20 μM)与MLL1 SET结构域(10 μM)在缓冲液(20 mM Tris-HCl pH 7.5、150 mM NaCl、1 mM DTT)中混合形成MLL1-WDR5复合物;将MM-102(50 μM,同缓冲液)在25°C下滴定至复合物中,记录热量变化,数据拟合1:1结合模型计算Ki[1] 2. MLL1-WDR5抑制AlphaScreen实验:生物素化MLL1肽段(3765–3785位氨基酸,50 nM)与GST标签WDR5(50 nM)在实验缓冲液(50 mM HEPES pH 7.4、100 mM NaCl、0.1% BSA)中,与系列浓度MM-102(0.1 nM–100 nM)室温孵育1 h;加入抗生物素供体微球和抗GST受体微球,检测615 nm荧光,从量效曲线推导IC₅₀[1] 3. MLL1甲基转移酶活性实验:重组MLL1复合物(MLL1-WDR5-Ash2L-RbBP5,各10 nM)与组蛋白H3(1–21)肽段(2 μM)、S-腺苷-L-甲硫氨酸(SAM,10 μM)及MM-102(0.1 nM–10 μM)37°C孵育2 h;用抗H3K4me3抗体通过ELISA检测H3K4me3水平,计算相对于对照组的抑制率,确定IC₅₀[1] 4. MLL1-WDR5结合HTRF实验(肾脏方向):荧光素标记MLL1肽段(50 nM)与WDR5(50 nM)在HTRF缓冲液(50 mM Tris-HCl pH 7.5、100 mM NaCl)中,与MM-102(0.1 nM–100 nM)孵育1 h;加入抗荧光素穴状化合物抗体和抗WDR5 d2抗体,检测时间分辨荧光,从量效曲线计算IC₅₀[2] |
| 细胞实验 |
HOXA9和MEIS-1基因的qRT-PCR分析[1]
用MLL1-AF9致癌基因转染正常小鼠骨髓细胞,获得MLL1-AF9转化的小鼠骨髓细胞,方法由Tan等描述。 MM-102和C-MM-102溶解于DMSO中。转化后的细胞分别用MM-102 (25 μM, 50 μM)、C-MM-102 (50 μM)和Mock (0.2% DMSO)处理,所有样品的终浓度均为0.2% DMSO。用Trizol和RNEASY试剂盒按照前面描述的方法处理96小时后,从MLL1-AF9转导的小鼠骨髓细胞中分离总RNA利用SuperScript III试剂盒随机引物生成cDNA。在SYBR染料存在的情况下,用每种基因特异性引物对HoxA9、Meis1和GAPDH基因进行实时PCR扩增。每个基因转录物的相对定量按照我们之前的工作进行在归一化到内部负载控制(例如,GAPDH或总输入RNA)后,将结果呈现为模拟处理的相对表达。 白血病细胞系细胞生长和凋亡的研究[1] MV4;11, KOPN8和K562细胞是来自密歇根大学Jolanta Grembecka博士的慷慨馈赠。MV4;11、KOPN8和K562细胞在添加10%胎牛血清和100 U/L青霉素-链霉素的RPMI 1640培养基(ATCC)中培养,在37℃、5% CO2下培养。将细胞以5 × 105 /孔(1ml)的密度接种于12孔板中悬浮,用载体对照(DMSO, 0.2%)或MM-102处理7天。每2天更换一次培养基,并补充化合物。 1. 白血病细胞MTT抗增殖实验:MV4-11或RS4;11细胞以3×10³个/孔接种96孔板,过夜培养;加入系列浓度MM-102(0.01 μM–20 μM),37°C、5% CO₂孵育72 h;每孔加MTT试剂(5 mg/mL,10 μL)孵育4 h,加二甲亚砜(100 μL/孔)溶解甲臜,检测570 nm吸光度,非线性回归计算IC₅₀[1] 2. 白血病细胞表观遗传与凋亡标志物Western blot实验:MV4-11细胞用MM-102(0.5–2 μM)处理48 h,提取核蛋白检测H3K4me3/HOXA9,提取总蛋白检测凋亡标志物;样品经SDS-PAGE电泳后转移至PVDF膜,用一抗(抗H3K4me3、抗HOXA9)和HRP偶联二抗孵育,信号相对于GAPDH定量[1] 3. 靶基因启动子区H3K4me3 ChIP-qPCR实验:1 μM MM-102处理48 h的MV4-11细胞用1%甲醛交联,超声破碎染色质,与抗H3K4me3抗体孵育,蛋白A/G珠捕获免疫复合物;纯化DNA后,用HOXA9/MEIS1启动子特异性引物进行qPCR[1] 4. 肾小管细胞凋亡实验:HK-2细胞用MM-102(1–5 μM)预处理2 h,再暴露于顺铂(20 μM)48 h;细胞用Annexin V-FITC和PI室温避光染色15 min,流式细胞术分析,计数凋亡细胞(Annexin V⁺/PI⁻ + Annexin V⁺/PI⁺)[2] 5. 肾小管细胞标志物Western blot实验:HK-2细胞按上述处理后提取总蛋白,膜用抗E-钙粘蛋白、抗磷酸化p53(Ser15)、抗切割型caspase-3和抗GAPDH抗体孵育,ImageJ软件定量条带强度[2] 6. 肾小管细胞基因表达qRT-PCR实验:处理后的HK-2细胞用TRIzol提取总RNA,逆转录为cDNA,用Bax、Hoxa9和GAPDH的特异性引物进行qPCR,2^(-ΔΔCt)法计算相对mRNA水平[2] |
| 动物实验 |
Animals models of AKI and treatment[2]
Male C57BL/6J mice aged 6–8 weeks and weighing 20–25 g were purchased from the Jackson Laboratory. The mice were randomly divided into four groups: (1) control, (2) MM-102, (3) cisplatin, and (4) MM-102 plus cisplatin. Cisplatin was intraperitoneally injected at the dose of 20 mg/kg. MM-102 (15 mg/kg) dissolved in solvent containing 10% DMSO and 90% corn oil was administered intraperitoneally 2 h before the cisplatin injection and then given daily for three consecutive days. The dose of MM-102 was selected according to a previous report. For the control and cisplatin-alone groups, mice were injected with an equivalent amount of solvent. Mice in the control and MM-102 groups were injected with an equal volume of a normal saline solution. All the mice were euthanized 72 h after cisplatin injection. Blood samples and kidney tissues were collected for further analysis. All experimental protocols were performed according to the National Institutes of Health Guidelines on the Care and Use of Laboratory Animals and approved by the Lifespan Animal Welfare Committee. The authorization number for the use of laboratory animals is 5074-19. 1. Leukemia subcutaneous xenograft model: Female NOD/SCID mice (6–8 weeks old) were subcutaneously injected with 5×10⁶ MV4-11 cells (PBS:Matrigel = 1:1) into the right flank. When tumors reached 100–150 mm³, mice were randomized into vehicle (n=6) and MM-102 (n=6) groups. MM-102 was dissolved in DMSO:PEG400:0.9% saline (15:35:50, v/v/v) to 4 mg/mL. Mice received 20 mg/kg MM-102 via oral gavage once daily for 21 days; vehicle received the same volume of solvent. Tumor volume (length × width² / 2) and body weight were measured every 3 days. Tumors were collected at sacrifice for molecular analysis [1] 2. Leukemia disseminated xenograft model: Female NOD/SCID mice were intravenously injected with 2×10⁶ MV4-11 cells via tail vein. Three days later, mice were randomized into vehicle (n=8) and MM-102 (n=8) groups. MM-102 was administered as above (20 mg/kg, oral gavage, qd) for 21 days. Mice were monitored for morbidity (weight loss >20%, lethargy), and survival time was recorded [1] 3. Cisplatin-induced AKI mouse model: Male C57BL/6 mice (8–10 weeks old) were divided into 3 groups (n=6/group): control, cisplatin alone, cisplatin + MM-102. MM-102 was dissolved in DMSO:corn oil (10:90, v/v) to 2 mg/mL. Mice in the combination group received 10 mg/kg MM-102 via intraperitoneal injection 1 h before cisplatin (20 mg/kg, intraperitoneal injection). Control mice received vehicle. On day 7 post-cisplatin, blood was collected for creatinine/BUN measurement, and kidneys were harvested for histopathology and Western blot [2] |
| 药代性质 (ADME/PK) |
1. Oral bioavailability in mice: Female CD-1 mice were given MM-102 via oral gavage (20 mg/kg) or intravenous injection (5 mg/kg). Blood samples were collected at 0.25, 0.5, 1, 2, 4, 8, 24 h post-dosing. Plasma MM-102 concentrations were measured by LC-MS/MS. Oral bioavailability was calculated as ~42% (AUC₀₋∞ oral / AUC₀₋∞ IV × dose IV / dose oral × 100%) [1]
2. Plasma pharmacokinetics (oral): After oral administration of 20 mg/kg MM-102 to CD-1 mice, key parameters were: Cₘₐₓ = ~3.1 μM, Tₘₐₓ = ~1.2 h, t₁/₂ = ~3.5 h, AUC₀₋₂₄ₕ = ~10.8 μM·h [1] 3. Tissue distribution: Tumor-bearing NOD/SCID mice (MV4-11 xenografts) were dosed with 20 mg/kg MM-102 (oral gavage). At 1.2 h post-dosing (Tₘₐₓ), tissues were collected and analyzed by LC-MS/MS. Concentrations were: tumor = ~2.8 μM, liver = ~4.5 μM, spleen = ~3.8 μM, lung = ~2.2 μM, kidney = ~1.9 μM. Tumor concentrations exceeded the in vitro IC₅₀ for MV4-11 cells (0.8 μM) [1] 4. Renal tissue distribution (AKI model): C57BL/6 mice were dosed with 10 mg/kg MM-102 (intraperitoneal injection). At 1 h post-dosing, kidney tissue MM-102 concentration was ~1.5 μM (LC-MS/MS), sufficient to inhibit MLL1-WDR5 interaction [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. Acute toxicity in mice: Female CD-1 mice were administered MM-102 via oral gavage at 50, 100, 150, 200 mg/kg. No mortality or overt toxicity (e.g., weight loss, lethargy) was observed at 200 mg/kg. LD₅₀ was determined to be >200 mg/kg [1]
2. Chronic toxicity in leukemia models: In the 21-day oral gavage study (20 mg/kg), MM-102-treated mice had no significant weight loss (max change: -5% vs. vehicle). Serum biochemical parameters (ALT, AST, creatinine, urea) were normal, and hematology (WBC, RBC, platelets) showed no abnormalities [1] 3. Toxicity in AKI model: Mice treated with MM-102 (10 mg/kg, i.p.) + cisplatin had no additional liver/kidney toxicity compared to cisplatin alone. Serum ALT/AST levels were normal, and kidney histopathology showed no MM-102-induced damage. No changes in body weight or behavior were observed [2] 4. Plasma protein binding: MM-102 (1 μM) was incubated with mouse plasma at 37°C for 1 h. Unbound drug was separated by ultrafiltration (30 kDa cutoff) and measured by LC-MS/MS. Plasma protein binding rate was ~93% [1][2] |
| 参考文献 |
|
| 其他信息 |
Mixed lineage leukemia 1 (MLL1) is a histone H3 lysine 4 (H3K4) methyltransferase, and targeting the MLL1 enzymatic activity has been proposed as a novel therapeutic strategy for the treatment of acute leukemia harboring MLL1 fusion proteins. The MLL1/WDR5 protein-protein interaction is essential for MLL1 enzymatic activity. In the present study, we designed a large number of peptidomimetics to target the MLL1/WDR5 interaction based upon -CO-ARA-NH-, the minimum binding motif derived from MLL1. Our study led to the design of high-affinity peptidomimetics, which bind to WDR5 with K(i) < 1 nM and function as potent antagonists of MLL1 activity in a fully reconstituted in vitro H3K4 methyltransferase assay. Determination of co-crystal structures of two potent peptidomimetics in complex with WDR5 establishes their structural basis for high-affinity binding to WDR5. Evaluation of one such peptidomimetic, MM-102, in bone marrow cells transduced with MLL1-AF9 fusion construct shows that the compound effectively decreases the expression of HoxA9 and Meis-1, two critical MLL1 target genes in MLL1 fusion protein mediated leukemogenesis. MM-102 also specifically inhibits cell growth and induces apoptosis in leukemia cells harboring MLL1 fusion proteins. Our study provides the first proof-of-concept for the design of small-molecule inhibitors of the WDR5/MLL1 protein-protein interaction as a novel therapeutic approach for acute leukemia harboring MLL1 fusion proteins.[1]
Mixed lineage leukemia 1 (MLL1) is a histone H3 lysine 4 (H3K4) methyltransferase that interacts with WD repeat domain 5 (WDR5) to regulate cell survival, proliferation, and senescence. The role of MLL1 in the pathogenesis of acute kidney injury (AKI) is unknown. In this study, we demonstrate that MLL1, WDR5, and trimethylated H3K4 (H3K4me3) were upregulated in renal tubular cells of cisplatin-induced AKI in mice, along with increased phosphorylation of p53 and decreased expression of E-cadherin. Administration of MM102, a selective MLL1/WDR5 complex inhibitor, improved renal function and attenuated tubular injury and apoptosis, while repressing MLL1, WDR5, and H3K4me3, dephosphorylating p53 and preserving E-cadherin. In cultured mouse renal proximal tubular cells (RPTCs) exposed to cisplatin, treatment with MM102 or transfection with siRNAs for either MLL1 or WDR5 also inhibited apoptosis and p53 phosphorylation while preserving E-cadherin expression; p53 inhibition with Pifithrin-α lowered cisplatin-induced apoptosis without affecting expression of MLL1, WDR5, and H3K4me3. Interestingly, silencing of E-cadherin offset MM102's cytoprotective effects, but had no effect on p53 phosphorylation. These findings suggest that MLL1/WDR5 activates p53, which, in turn, represses E-cadherin, leading to apoptosis during cisplatin-induced AKI. Further studies showed that MM102 effectively inhibited cisplatin-triggered DNA damage response (DDR), as indicated by dephosphorylation of ataxia telangiectasia mutated (ATM) and ATM and Rad-3 related (ATR) proteins, dephosphorylation of checkpoint kinase 1 and 2 (Chk1 and Chk2); depression of γ-H2AX; and restrained cell cycle arrest, as evidenced by decreased expression of p21 and phospho-histone H3 at serine 10 in vitro and in vivo. Overall, we identify MLL1 as a novel DDR regulator that drives cisplatin-induced RPTC apoptosis and AKI by modulating the MLL1/WDR5-/ATR/ATM-Chk-p53-E-cadherin axis. Targeting the MLL1/WDR5 complex may have a therapeutic potential for the treatment of AKI.[2] 1. Mechanism of action: MM-102 is a peptidomimetic inhibitor of the MLL1-WDR5 protein-protein interaction. It binds to the "WIN" site of WDR5, blocking MLL1 recruitment to WDR5 and disrupting the MLL1 methyltransferase complex. This inhibits H3K4me3 at MLL1 target genes (e.g., HOXA9, MEIS1), suppressing oncogenic transcription in leukemia and p53-mediated apoptosis in cisplatin-induced AKI [1][2] 2. Therapeutic background in leukemia: MLL-rearranged leukemias depend on MLL1-WDR5 interaction for oncogene expression and cell survival. MM-102 targets this dependency, showing preclinical efficacy in xenograft models, supporting its development for MLL-rearranged leukemia treatment [1] 3. Therapeutic potential in AKI: Cisplatin-induced AKI involves MLL1-mediated repression of E-cadherin and activation of p53-dependent apoptosis. MM-102 inhibits MLL1, restores E-cadherin expression, and reduces tubular cell apoptosis, representing a novel therapeutic strategy for cisplatin-induced kidney injury [2] 4. Peptidomimetic design: MM-102 was designed based on the MLL1 "WIN" motif peptide, with structural modifications (e.g., cyclization, hydrophobic substitutions) to enhance binding affinity, stability, and oral bioavailability—key advantages over linear peptide inhibitors [1] |
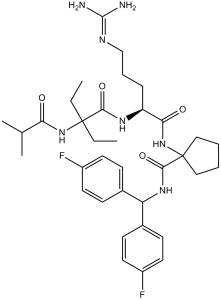
| 分子式 |
C35H49F2N7O4
|
|
|---|---|---|
| 分子量 |
669.8
|
|
| 精确质量 |
669.381
|
|
| 元素分析 |
C, 62.76; H, 7.37; F, 5.67; N, 14.64; O, 9.55
|
|
| CAS号 |
1417329-24-8
|
|
| 相关CAS号 |
MM-102 TFA;1883545-52-5
|
|
| PubChem CID |
54766613
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| LogP |
6.433
|
|
| tPSA |
178.3
|
|
| 氢键供体(HBD)数目 |
6
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
16
|
|
| 重原子数目 |
48
|
|
| 分子复杂度/Complexity |
1080
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
O=C(C1(NC([C@@H](NC(C(NC(C(C)C)=O)(CC)CC)=O)CCCNC(N)=N)=O)CCCC1)NC(C2=CC=C(F)C=C2)C3=CC=C(F)C=C3
|
|
| InChi Key |
RZKSQRIPRKWVBU-MHZLTWQESA-N
|
|
| InChi Code |
InChI=1S/C35H49F2N7O4/c1-5-34(6-2,43-29(45)22(3)4)31(47)41-27(10-9-21-40-33(38)39)30(46)44-35(19-7-8-20-35)32(48)42-28(23-11-15-25(36)16-12-23)24-13-17-26(37)18-14-24/h11-18,22,27-28H,5-10,19-21H2,1-4H3,(H,41,47)(H,42,48)(H,43,45)(H,44,46)(H4,38,39,40)/t27-/m0/s1
|
|
| 化学名 |
N-[Bis(4-fluorophenyl)methyl]-1-[[(2S)-5-(diaminomethylideneamino)-2-[[2-ethyl-2-(2-methylpropanoylamino)butanoyl]amino]pentanoyl]amino]cyclopentane-1-carboxamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4930 mL | 7.4649 mL | 14.9298 mL | |
| 5 mM | 0.2986 mL | 1.4930 mL | 2.9860 mL | |
| 10 mM | 0.1493 mL | 0.7465 mL | 1.4930 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01997840 | Active, not recruiting | Drug: ACY-1215 (Ricolinostat) in combination with pomalidomide and dexamethasone |
Multiple Myeloma | Celgene | March 1, 2014 | Phase 1 Phase 2 |
| NCT02426723 | Completed | Drug: Phase 1a: CWP232291
Drug: Phase 1b: CWP232291, Lenalidomide, Dexamethasone |
Multiple Myeloma |
JW Pharmaceutical | October 19, 2015 | Phase 1 |
| NCT00985959 | Completed Has Results | Drug: JNJ-26866138 0.7 mg/m2 Drug: JNJ-26866138 1.0 mg/m2 |
Multiple Myeloma |
Janssen Pharmaceutical K.K. | July 2008 | Phase 1 |
 |
|---|