| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
| 靶点 |
SIRT2 (EC50 = 2 μM); SIRT1 (EC50 = 50~180 μM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:烟酰胺强烈抑制酵母沉默,增加 rDNA 重组,并缩短 Sir2 突变体的复制寿命。即使在 G(1) 停滞的细胞中,烟酰胺也会消除沉默并导致 Sir2 最终离域,这表明沉默的异染色质需要持续的 Sir2 活性。烟酰胺导致胎儿细胞中 DNA 含量增加两倍,胰岛素含量增加三倍。烟酰胺诱导人胎儿胰岛细胞的分化和成熟。烟酰胺通过在脱乙酰化和碱基交换之间切换来调节去乙酰化酶。对来自古球菌 (Sir2Af2)、酿酒酵母 (Sir2p) 和小鼠 (Sir2alpha) 的 Sir2s 的烟酰胺转换进行定量。烟酰胺以类似于抑制 SirT1 的方式选择性地减少与阿尔茨海默病转基因小鼠中微管解聚相关的 tau 蛋白 (Thr231) 的特定磷酸种类。烟酰胺还显着增加阿尔茨海默病转基因小鼠中乙酰化 α-微管蛋白(SirT2 的主要底物)和 MAP2c,这两者都与增加微管稳定性有关。烟酰胺促进 DNA 完整性并维持磷脂酰丝氨酸膜不对称性,以防止细胞炎症、细胞吞噬作用和血管血栓形成。烟酰胺可以预防和逆转神经元和血管细胞损伤。细胞测定:先前的研究结果表明烟酰胺对 PARP-1 诱导的星形胶质细胞死亡具有保护作用。转运蛋白介导的烟酰胺摄取对细胞外 pH 值敏感,并且是 N-甲基烟酰胺所共有的,被发现对于预防 PARP-1 触发的细胞死亡至关重要。
|
||
| 体内研究 (In Vivo) |
在 Wistar 大鼠中先使用链脲佐菌素,然后使用烟酰胺诱导 2 型糖尿病。测试化合物和标准治疗持续15天。结果显示,与糖尿病大鼠相比,肝脏抗氧化酶显着正常化,表明所有测试的化合物都有益于减少氧化应激
|
||
| 酶活实验 |
酿酒酵母Sir2蛋白是一种NAD(+)依赖性组蛋白脱乙酰酶,在转录沉默、基因组稳定性和寿命方面发挥着关键作用。Sir2的人类同源物SIRT1调节p53肿瘤抑制因子的活性并抑制细胞凋亡。Sir2脱乙酰反应产生两种产物:O-乙酰-ADP-核糖和烟酰胺,烟酰胺是烟酸的前体和烟酸/维生素B的一种形式(3)。我们在这里表明,烟酰胺强烈抑制酵母沉默,增加rDNA重组,并将复制寿命缩短到sir2突变体的寿命。烟酰胺消除了沉默,并导致Sir2最终离域,即使在G(1)停滞的细胞中也是如此,这表明沉默的异染色质需要持续的Sir2活性。我们发现生理浓度的烟酰胺在体外非竞争性地抑制Sir2和SIRT1。烟酰胺的抑制程度(IC(50)<50微米)等于或优于这类蛋白质的已知最有效的合成抑制剂。我们提出了一种模型,烟酰胺通过与NAD(+)附近的保守口袋结合来抑制脱乙酰化,从而阻断NAD(+)水解。我们讨论了烟酰胺是Sir2酶的生理相关调节因子的可能性。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
14(C)Niacinamide was incorporated into an oil-in-water (o/w) skin cream and into a 30% (w/w) soap base and applied to the skin of female Colworth Wistar rats. The final concentration of niacinamide in the soap solution was approximately 0.3% (w/v) and was 1% (w/w) in the skin cream. Application of the skin cream and soap paste was made to rat skin at approximately 20 mg/sq cm. The cream was carefully massaged over 10 sq cm of skin for up to 5 min before covering with polythene-lined occlusive protective patches. The rats were placed in metabolism cages for 48 hr during which time all excreta was collected. At 48 hr, the animals were killed and the patch, carcass, and treated area of skin were assayed for 14(C). Up to 32% 14(C) was recovered in excreta and in the carcasses from rats treated with skin cream containing 14(C)Niacinamide and up to 30% from those treated with soap paste. Nicotinamide is efficiently absorbed from the gastrointestinal tract. At low doses, absorption is mediated via sodium-dependent facilitated diffusion. Passive diffusion is the principal mechanism of absorption at higher doses. Doses of up to three to four grams of nicotinamide are almost completely absorbed. Nicotinamide is transported via the portal circulation to the liver and via the systemic circulation to the various tissues of the body. Nicotinamide enters most cells by passive diffusion and enters erythrocytes by facilitated transport. Niacinamide is widely distributed /throughout/ body tissues. Niacin and niacinamide are readily absorbed from the GI tract following oral administration, and niacinamide (no longer commercially available in the US) is readily absorbed from subcutaneous and IM injection sites. For more Absorption, Distribution and Excretion (Complete) data for Nicotinamide (16 total), please visit the HSDB record page. Metabolism / Metabolites In amounts needed for physiologic function as a coenzyme (12-18 mg daily), niacin is converted to niacinamide; larger doses of niacin are converted to niacinamide to only a minor degree. Niacinamide is metabolized in the liver to N-methylniacinamide, other N-methylated derivatives, and nicotinuric acid (the glycine conjugate of niacin). These metabolites are excreted in urine. Following administration of physiologic doses of niacin or niacinamide, only a small amount of niacinamide is excreted unchanged in urine; however, following administration of larger doses, a greater proportion of niacin and niacinamide is excreted unchanged. N1-Methyl-4-pyridone-3-carboxamide was detected on chromatograms of plasma extracts after oral administration of niacinamide to two human subjects. 6-Hydroxynicotinamide and 6-hydroxynicotinic acid /were detected/ as urinary metabolites by comparison of ultraviolet, infrared, and mass spectra following intraperitoneal injections of 14(C)Niacin or 14(C)Niacinamide into rats. N1-methyl-4- pyridone-3-carboxamide is a major metabolite of niacin and niacinamide which has been found to be synthesized from N1- methylnicotinamide. For more Metabolism/Metabolites (Complete) data for Nicotinamide (7 total), please visit the HSDB record page. Uremic toxins tend to accumulate in the blood either through dietary excess or through poor filtration by the kidneys. Most uremic toxins are metabolic waste products and are normally excreted in the urine or feces. Biological Half-Life The mean half life values were 2.7 hr, 5.9 hr, and 8.1 hr after taking 1, 3, or 6 g of Niacinamide, respectively. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Nicotinamide is a white, crystalline powder. Nicotinamide is used to prevent niacin deficiency and to treat pellagra. Nicotinamide is also used in cosmetics as a hair and skin conditioning agent. It has been used in the enrichment of bread, flour, and other grain-derived products. Animal feed is routinely supplemented with nicotinamide. It is also used in multi-vitamin preparations. Nicotinamide and niacin are similarly effective as a vitamin because they can be converted into each other within the organism. The blanket term vitamin B(3) is used for both. Niacinamide is a component of important coenzymes involved in hydrogen transfer. HUMAN STUDIES: In humans, nicotinamide is required for lipid metabolism, tissue respiration, and glycogenolysis. In vivo, nicotinamide is formed from conversion of niacin. In addition, some dietary tryptophan is oxidized to niacin and then to nicotinamide in vivo. Nicotinamide is incorporated into 2 coenzymes: nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). NAD and NADP act as hydrogen-carrier molecules in glycogenolysis, tissue respiration, and lipid metabolism. In a study with 6 volunteers (single dose between 3 and 9 g/day) toxic symptoms associated with nicotinamide were mild and consisted mainly of nausea. The effect of 2 mM nicotinamide on unscheduled DNA synthesis on resting human lymphocytes was studied. In cells treated with UV irradiation or with N-methyl-N-nitro- N-nitrosoguanidine, nicotinamide caused a two-fold stimulation of unscheduled DNA synthesis. ANIMAL STUDIES: Application of 0.1 g nicotinamide to the eyes of rabbits induced reversible irritation. It did not produced sensitization in guinea pig test. Single ip injection of nicotinamide (100 mg/kg) to male rats was shown to significantly induce all components of the hepatic microsomal mixed function oxidase system as well as activities of drug-metabolizing enzymes. Groups of 12 male rats were fed nicotinamide in their diet for a period of 8 to 12 weeks. At 0.1% of the diet (100 mg/kg bw per day), nicotinamide caused no significant change in the growth rate, at 0.2%, growth rate was enhanced, but at 0.4%, a marked inhibition of growth rate resulted. Almost complete growth inhibition occurred in rats fed 1% nicotinamide. Lifelong treatment with 1% nicotinamide had no carcinogenic effect in mice. However, nicotinamide promoted diethylnitrosamine-induced renal tubular cell tumorigenesis in rats. In mice nicotinamide in doses of 500-2000 mg/kg depresses orientation reflexes and exploring behavior, and has antiaggressive and anticonvulsant properties. Nicotinamide supplementation in pregnant rats led to a decrease in placental and fetal hepatic genomic DNA methylation and genomic uracil contents (a factor modifying DNA for diversity) in the placenta and fetal liver and brain, which could be completely or partially prevented by betaine. Moreover, nicotinamide supplementation induced tissue-specific alterations in the mRNA expression of the genes encoding nicotinamide N-methyltransferase, DNA methyltransferase 1, catalase and tumor protein p53 in the placenta and fetal liver. High-dose nicotinamide supplementation increased fetal hepatic a-fetoprotein mRNA level, which was prevented by betaine supplementation. It is concluded that maternal nicotinamide supplementation can induce changes in fetal epigenetic modification and DNA base composition. Nicotinamide was negative in an Ames test performed with Salmonella strains TA 98, TA 100, TA 1535, TA 1537 and TA 1538 both with and without metabolic activation. Nicotinamide was not mutagenic in Saccharomyces stain D4. It was reported that nicotinamide at concentrations of 3 mg/mL (25 mM) induced large structural chromosome aberrations in vitro in Chinese hamster ovary cells. Uremic toxins such as nicotinamide are actively transported into the kidneys via organic ion transporters (especially OAT3). Increased levels of uremic toxins can stimulate the production of reactive oxygen species. This seems to be mediated by the direct binding or inhibition by uremic toxins of the enzyme NADPH oxidase (especially NOX4 which is abundant in the kidneys and heart) (A7868). Reactive oxygen species can induce several different DNA methyltransferases (DNMTs) which are involved in the silencing of a protein known as KLOTHO. KLOTHO has been identified as having important roles in anti-aging, mineral metabolism, and vitamin D metabolism. A number of studies have indicated that KLOTHO mRNA and protein levels are reduced during acute or chronic kidney diseases in response to high local levels of reactive oxygen species (A7869). Interactions Addition of 0.5 mg of nicotinamide reduced the action of dicrotophos on cultured chick embryo tibiae. Nicotinamide will prevent depletion of NAD coenzymes by alkylating agents. Renal oncogenic activity of Streptozotocin in male rats was significantly decreased by nicotinamide. Oral or iv administered nicotinamide prevented Streptozotocin-induced diabetes in Rhesus monkeys and dogs. For more Interactions (Complete) data for Nicotinamide (25 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 3500 mg/kg LD50 Rat sc 1680 mg/kg LD50 Mouse oral 2500 mg/kg LD50 Mouse ip 2050 mg/kg For more Non-Human Toxicity Values (Complete) data for Nicotinamide (9 total), please visit the HSDB record page. |
||
| 参考文献 |
J Biol Chem.2002 Nov 22;277(47):45099-107.
|
||
| 其他信息 |
Nicotinamide is a white powder. (NTP, 1992)
Nicotinamide is a pyridinecarboxamide that is pyridine in which the hydrogen at position 3 is replaced by a carboxamide group. It has a role as an EC 2.4.2.30 (NAD(+) ADP-ribosyltransferase) inhibitor, a metabolite, a cofactor, an antioxidant, a neuroprotective agent, an EC 3.5.1.98 (histone deacetylase) inhibitor, an anti-inflammatory agent, a Sir2 inhibitor, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a mouse metabolite, a human urinary metabolite and a geroprotector. It is a vitamin B3, a pyridinecarboxamide and a pyridine alkaloid. It is functionally related to a nicotinic acid. An important compound functioning as a component of the coenzyme NAD. Its primary significance is in the prevention and/or cure of blacktongue and pellagra. Most animals cannot manufacture this compound in amounts sufficient to prevent nutritional deficiency and it therefore must be supplemented through dietary intake. Niacinamide is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Nicotinamide has been reported in Lactarius subplinthogalus, Taraxacum formosanum, and other organisms with data available. Niacinamide is the active form of vitamin B3 and a component of the coenzyme nicotinamide adenine dinucleotide (NAD). Niacinamide acts as a chemo- and radio-sensitizing agent by enhancing tumor blood flow, thereby reducing tumor hypoxia. This agent also inhibits poly(ADP-ribose) polymerases, enzymes involved in the rejoining of DNA strand breaks induced by radiation or chemotherapy. Nicotinamide is a uremic toxin. Uremic toxins can be subdivided into three major groups based upon their chemical and physical characteristics: 1) small, water-soluble, non-protein-bound compounds, such as urea; 2) small, lipid-soluble and/or protein-bound compounds, such as the phenols and 3) larger so-called middle-molecules, such as beta2-microglobulin. Chronic exposure of uremic toxins can lead to a number of conditions including renal damage, chronic kidney disease and cardiovascular disease. Niacinamide or vitamin B3 is an important compound functioning as a component of the coenzyme NAD. Its primary significance is in the prevention and/or cure of blacktongue and pellagra. Most animals cannot manufacture this compound in amounts sufficient to prevent nutritional deficiency and it therefore must be supplemented through dietary intake. Niacinamide is used to increase the effect of radiation therapy on tumor cells. Niacin (nicotinic acid) and niacinamide, while both labeled as vitamin B3 also have different applications. Niacinamide is useful in arthritis and early-onset type I diabetes while niacin is an effective reducer of high cholesterol levels. Niacinamide is a metabolite found in or produced by Saccharomyces cerevisiae. An important compound functioning as a component of the coenzyme NAD. Its primary significance is in the prevention and/or cure of blacktongue and PELLAGRA. Most animals cannot manufacture this compound in amounts sufficient to prevent nutritional deficiency and it therefore must be supplemented through dietary intake. See also: Niacinamide ascorbate (is active moiety of); Dapsone; niacinamide (component of); Adenosine; Niacinamide (component of) ... View More ... Therapeutic Uses Vitamin B Complex /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Nicotinamide is included in the database. Niacin and niacinamide are used to prevent niacin deficiency and to treat pellagra. Some clinicians prefer niacinamide for the treatment of pellagra because it lacks vasodilating effects. Pellagra may result from dietary deficiency, isoniazid therapy, or from decreased conversion of tryptophan to niacin in Hartnup disease or carcinoid tumors. /Included in US product label/ Although niacin and niacinamide have not been shown by well-controlled trials to have therapeutic value, the drugs have been used for the management of schizophrenic disorder, drug-induced hallucinations, chronic brain syndrome, hyperkinesis, unipolar depression, motion sickness, alcohol dependence, livedoid vasculitis, acne, and leprosy. /NOT included in US product label/ For more Therapeutic Uses (Complete) data for Nicotinamide (14 total), please visit the HSDB record page. Drug Warnings Blood glucose concentration should be monitored periodically in patients receiving niacin or niacinamide, especially early in the course of therapy. Dosage requirements for antidiabetic agents (e.g., insulin, oral sulfonylureas) may change in diabetic patients. Potential adverse effects on fetus: Higher levels in fetus than mother, but no fetal anomalies reported. Potential side effects on breast-fed infant: No adverse effects known . FDA Category: C (C = Studies in laboratory animals have revealed adverse effects on the fetus (teratogenic, embryocidal, etc.), but there are no controlled studies in pregnant women. The benefits from use of the drug in pregnant women may be acceptable despite its potential risks, or there are no laboratory animal studies or adequate studies in pregnant women.) /from table II/ Niacinamide /was administered/ daily as a liquid formulation to head and neck cancer patients receiving a 5- to 7-week course of radiotherapy. Niacinamide was administered orally 1.5 hr before irradiation. The daily dose was 80 mg/kg bw to a maximum of 6 g. A dose reduction to 60 mg/kg was introduced for patients with severe side-effects. ... Side-effects of niacinamide were monitored. In all patients, peak concentrations greater than 700 nM/mL could be obtained 0.25-3 hr after drug intake. During the first week of treatment, plasma concentrations at the time of irradiation were adequate in 82% of the samples. Nausea, with or without vomiting, occurred in 65% of patients. Tolerance improved after a 25% reduction of the dose in six of seven patients but plasma concentrations at the time of irradiation decreased below 700 nM/mL in four out of six patients. Other niacinamide side effects included gastrointestinal symptoms, flushing, dizziness, sweating, fatigue, and headache. The most powerful single predictor for severe niacinamide toxicity was the mean of the plasma concentration measured at the time of irradiation during the first week. Abnormal liver function test results (including increased serum concentrations of bilirubin, AST [SGOT], ALT [SGPT], and LDH), jaundice, and chronic liver damage have occurred during niacin and niacinamide therapy. Abnormal prothrombin time and hypoalbuminemia have also been reported. For more Drug Warnings (Complete) data for Nicotinamide (6 total), please visit the HSDB record page. |
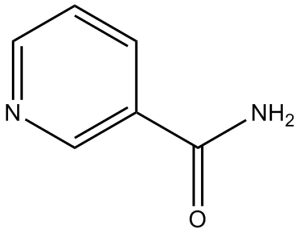
| 分子式 |
C6H6N2O
|
|
|---|---|---|
| 分子量 |
122.12
|
|
| 精确质量 |
122.048
|
|
| 元素分析 |
C, 59.01; H, 4.95; N, 22.94; O, 13.10
|
|
| CAS号 |
98-92-0
|
|
| 相关CAS号 |
25334-23-0; Nicotinamide;98-92-0
|
|
| PubChem CID |
936
|
|
| 外观&性状 |
White to off-white solid
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
257.7±32.0 °C at 760 mmHg
|
|
| 熔点 |
128-131 °C(lit.)
|
|
| 闪点 |
109.7±25.1 °C
|
|
| 蒸汽压 |
0.0±0.6 mmHg at 25°C
|
|
| 折射率 |
1.590
|
|
| 来源 |
Endogenous Metabolite
|
|
| LogP |
-0.24
|
|
| tPSA |
55.98
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
9
|
|
| 分子复杂度/Complexity |
114
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=C(C1=C([H])N=C([H])C([H])=C1[H])N([H])[H]
|
|
| InChi Key |
DFPAKSUCGFBDDF-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C6H6N2O/c7-6(9)5-2-1-3-8-4-5/h1-4H,(H2,7,9)
|
|
| 化学名 |
3-Pyridinecarboxylic acid amide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.03.00
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 8.1887 mL | 40.9433 mL | 81.8867 mL | |
| 5 mM | 1.6377 mL | 8.1887 mL | 16.3773 mL | |
| 10 mM | 0.8189 mL | 4.0943 mL | 8.1887 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04843553 | Completed | Drug: Oral Nicotinamide | Actinic Keratoses | Rhode Island Hospital | October 14, 2016 | Early Phase 1 |
| NCT06007391 | Not yet recruiting | Drug: Nicotinamide | Nicotinamide Adverse Reaction | University Hospital, Angers | September 2023 | Phase 2 Phase 3 |
| NCT03789175 | Completed Has Results | Dietary Supplement: Nicotinamide Riboside (NR) |
Cancer Skin Fibroblasts |
National Heart, Lung, and Blood Institute (NHLBI) |
March 25, 2019 | Phase 1 Phase 2 |
| NCT03432871 | Completed | Dietary Supplement: Nicotinamide Riboside |
Mitochondrial Diseases Mitochondrial Myopathies |
Cambridge University Hospitals NHS Foundation Trust |
December 8, 2017 | Not Applicable |
|
|
|