| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
HIV-1 capsid
|
|---|---|
| 体外研究 (In Vitro) |
Lenacapavir 干扰 HIV-1 复制的早期和晚期阶段,但针对早期阶段更有效 [2]。 Lenacapavi (GS-6207) 是一种有效的 HIV 复制衣壳抑制剂。 Lenacapavi 在靶细胞 (EC50=23 pM)、全周期测定 (EC50=25 pM) 和生产细胞 (EC50=439 PM) 中表现良好。
在体外,LEN对SHIV表现出强大的抗病毒活性,就像对HIV-1一样。在猕猴中,单次皮下给药LEN显示出药物血浆水平的剂量比例增加和持久性。[3] |
| 体内研究 (In Vivo) |
通过病毒滴定在未经处理的猕猴中鉴定用于PrEP疗效评估的高剂量SHIV接种物。LEN治疗的猕猴在给药7周后接受高剂量SHIV攻击,血浆PCR、细胞相关前病毒DNA和血清学检测证实,大多数猕猴仍能免受感染。在激发时LEN血浆暴露量超过其模型调整的临床疗效目标的动物中,观察到完全保护和优于未治疗组。所有受感染的动物都具有亚保护性LEN浓度,并且没有表现出突发耐药性。这些数据证明了在临床相关LEN暴露的严格猕猴模型中有效预防SHIV,并支持LEN对人类HIV PrEP的临床评估。[3]
|
| 酶活实验 |
微尺度热电泳分析[1]
CA与Pep-1和PF74的结合亲和力是通过在Pep-1或PF74浓度增加的情况下测量荧光标记的CA六聚体的热泳来测定的。肽Pep-1是在分子相互作用核心中合成的,PF74是商业购买的。根据制造商的说明书(MO-L004 Monolith Protein labeling Kit),用Alexa Fluor 647类似物NT647对CA进行荧光标记。简言之,将20μM蛋白质与3M过量染料在室温下在标记试剂盒提供的缀合缓冲液中孵育过夜。通过与试剂盒一起提供的重力流柱过滤除去未反应的染料。将洗脱组分收集在2×MST缓冲液(40mM MOPS,pH 7.2,200mM NaCl和0.2%pluronic F-127)中。通过MST评估每个级分的荧光强度,并合并含有标记蛋白的级分。通过NanoDrop分光光度计测定蛋白质浓度。等分试样在使用前储存在−80°C下。将含有200 nM标记的CA六聚体和不断增加的Pep-1浓度(1–2000 nM)的反应混合物加载到毛细管中,并在20%LED功率、高MST功率和20 s MST准时下监测热泳。 体外HIV-1 CA组装测定。[2] 在存在和不存在小分子文库化合物(10μM)或2倍连续稀释的GS-6207的情况下,通过在350nm处测量样品吸光度随时间的变化来监测HIV-1 CA蛋白的体外组装。最终组装反应包含20μM CA、2M NaCl、50mM磷酸钠(pH 7.5)、0.005%消泡剂204(Sigma-Aldrich)和1%二甲基亚砜。在25°C下,使用M5读板器在96孔板或384孔板中监测350 nm处的样品吸光度值,在没有CA或NaCl的情况下校正吸光度值,并使用SoftMax Pro 6.3.1分析数据,如前所述38。 GS-6207结合测定。[2] 使用ProteOn XPR36平台(CA六聚体和五聚体蛋白)或Biacore T100平台(CA单体和Gag蛋白)进行表面等离子体共振生物传感器结合实验,如前所述21。数据使用ProteOn Manager 3.1.0或Scrubber 2.0进行分析,并符合一个简单的动力学模型,必要时添加了质量传输术语。 |
| 细胞实验 |
细胞毒性测定。[2]
对于MT-4细胞、PBMC、原代人CD4+T细胞和单核细胞衍生的巨噬细胞的细胞毒性评估,该方案与各自的抗病毒试验的方案相同,包括试验持续时间,只是没有向平板中添加病毒。先前已经描述了Huh-7、Gal-HepG2、Gal-PC-3和MRC-5细胞系以及原代人类肝细胞中的细胞毒性评估方案37。使用CellTiter Glo测量测试化合物对细胞活力的影响。使用GraphPad Prism 7.0进行数据分析以计算CC50值。 GS-6207电阻分析。[2] 如前所述,在感染HIV-1HXB2D的MT-2细胞中使用GS-6207浓度的两倍增量增加进行耐药HIV-1变体的剂量递增选择31。在滴定病毒接种物以使所有样本的m.o.i.正常化后,在5天的细胞保护抗病毒MT-2测定中评估每个新出现的病毒传代的抗性谱。如前所述,在固定、恒定药物浓度的条件下,在独立感染六种不同HIV-1分离株(BaL、92US657、91US0006、7406、7467和7576)的人PBMC中进行为期35天的病毒突破性选择21。在固定药物浓度下测试GS-6207,该固定药物浓度等于其0.23 nM的EC95值的4倍、8倍和16倍(分别为0.92 nM、1.9 nM和3.7 nM GS-6207),每个实验条件使用6个重复细胞培养物。在GS-6207存在的情况下出现的病毒通过群体测序进行基因分型。使用QiaAMP病毒RNA迷你试剂盒从模拟和GS-6207-选择的含病毒上清液中分离总RNA。使用Qiagen OneStep RT-PCR试剂盒与引物5’-CCAGTAGCAACCCTCTATTGTGC-3’和5’-CCCTAGGGCCCTCTAATT-3’组合,通过RT-PCR扩增编码HIV-1衣壳和相邻p2间隔肽的986bp片段。RT-PCR产物由Elim Biopharmaceuticals进行测序。为了鉴定密码子的变化,使用DNA Sequencer 4.9软件将所选HIV-1变体的基因序列与输入病毒和在没有GS-6207的情况下传代的病毒的基因序列进行比对。对于含有>1个密码子变化的样本,对PCR产物进行亚克隆,从单个菌落中分离DNA,并对CA基因进行测序,以评估所有观察到的取代的连锁。 |
| 动物实验 |
Drug and formulation.[3]
LEN and the liquid chromatography–mass spectrometry internal standard GS-224337 were synthesized internally. and subjected to a standard quality control analysis. For antiviral assays, LEN was dissolved in DMSO to produce a 10 mM stock concentration and stored frozen at –0°C. For animal dosing studies, LEN was dissolved in vehicle (58.03% polyethylene glycol 300, 27.1% water, 6.78% ethanol, 6.61% poloxamer 188, 1.48% sodium hydroxide) at 300 mg/mL, stored at ambient temperature, and protected from light until dosing. The formulation contained additional excipients absent from the clinical formulation in order to tailor the pharmacokinetic profile in macaques. Animal studies.[3] All animals were housed at Bioqual Inc. For the in vivo SHIV stock titration study, 8 untreated outbred Indian-origin male rhesus macaques aged 3–5 years were challenged intrarectally per round for a total of 5 challenge rounds using increasing virus doses ranging from 0.625 to 100 TCID50, with the 100 TCID50 round performed twice for increased resolution (Supplemental Table 2). Plasma viral load was measured to confirm the infection status. For LEN pharmacokinetics and PrEP efficacy determination, 20 outbred Indian-origin male rhesus macaques aged 3–5 years were assigned to 5 study groups with an even weight distribution (Supplemental Table 2). On study week 0, 4 animals per group were administered LEN at 5, 10, 20, 50, or 75 mg/kg in the scapular region by subcutaneous injection. LEN was prepared as a 300 mg/mL stock solution, and no more than 2 mL solution was injected into a single subcutaneous site. Injection sites were monitored daily by veterinary staff for 2 weeks and then weekly through the end of study. On week 7, 11 animals were challenged by the intrarectal route with 1 mL RPMI containing 100 TCID50 SHIV-SF162P3. Whole blood was collected and processed into plasma and PBMCs as necessary for the assessment of routine hematology and clinical chemistry, viral load analysis, serology, and the bioanalysis of drug levels. Animals were considered protected if they remained SHIV negative by a plasma PCR assay and seronegative by enzyme immunoassay through week 10 after challenge. Animals confirmed as SHIV positive in both the virus titration and the PrEP efficacy studies were placed on a daily subcutaneous ART regimen between weeks 4 and 10 after infection to prevent AIDS disease progression. The formulated ART cocktail contained tenofovir disoproxil fumarate (5.1 mg/mL), emtricitabine (40 mg/mL), and dolutegravir (2.5 mg/mL) and was administered subcutaneously once daily at 1 mL/kg. View More
Bioanalysis of LEN in macaque plasma.[3] Plasma viral load assay.[3] A QIAsymphony SP (Qiagen) automated sample preparation platform along with a Virus/Pathogen DSP midi kit and the cellfree500 protocol were used to extract viral RNA from 500 μL plasma. A reverse primer specific to the gag gene of SIVmac251 (5′-CACTAGGTGTCTCTGCACTATCTGTTTTG-3′) was annealed to the extracted RNA and then reverse transcribed into cDNA using SuperScript III Reverse Transcriptase along with RNAse Out (Thermo Fisher Scientific). The resulting cDNA was treated with RNase H (Thermo Fisher Scientific) and then added (2 replicates) to a custom 4× TaqMan Gene Expression Master Mix (Thermo Fisher Scientific) containing primers and a fluorescently labeled hydrolysis probe specific for the gag gene of SIVmac251 (forward primer 5′-GTCTGCGTCATCTGGTGCATTC-3′, reverse primer 5′-CACTAGGTGTCTCTGCACTATCTGTTTTG-3′, probe 5′-/56-FAM/CTTCCTCAGTGTGTTTCACTTTCTCTTCTGCG/3BHQ_1/-3′). The qPCR was then carried out on a QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific). Mean SIV gag RNA copies per reaction were interpolated using quantification cycle data and a serial dilution of a highly characterized custom RNA transcript containing a 730 bp sequence of the SIV gag gene. The assay limit of quantification is approximately 62 RNA copies per milliliter of sample. ELISA.[3] Rhesus serum samples from viremic study animals were tested for the presence of antibodies against HIV-1 by ELISA using the GS HIV-1/HIV-2 PLUS O EIA assay kit from Bio-Rad. Individual macaque sera (150 μL) mixed with 50 μL specimen diluent supplied in the kit were added to assay plates precoated with recombinant purified HIV-1 capsid (p24) protein and transmembrane glycoprotein (gp160) and incubated for 1 hour at room temperature. The plates were then washed 3 times with a sodium chloride and Tween 20–containing wash buffer from the kit and incubated for 1 hour with a HRP-conjugated antigen solution containing peptides mimicking various immunodominant epitopes of HIV-1 gp160 and p24 proteins. Wells with antibody against HIV-1 bound to the antigen coating the wells and to the peroxidase-conjugated antigens in the conjugate solution to form immobilized stable antigen-antibody-antigen complexes. The plates were washed 3 more times with the above wash buffer, developed with a working solution of tetramethylbenzidine, stopped by the addition of 1 N sulfuric acid, and analyzed at 450 nm using a Versamax microplate reader using the Softmax Pro 6.5.1 software. Samples with an OD450 nm absorbance value of more than 0.2 were considered positive. IPDA.[3] The SHIV-adapted version of IPDA (SHIV-IPDA) was used to determine the number of intact SHIV proviruses. Total genomic DNA was extracted from unfractionated PBMCs using a QIAamp DNA Mini kit. DNA quality and quantity were evaluated by spectrophotometry and fluorometry, respectively, and SHIV-IPDA was then performed on the isolated DNA. In brief, SHIV-IPDA consists of a 3-component multiplex droplet-digital PCR (ddPCR) reaction. The first is a SHIV proviral discrimination reaction targeting two conserved, frequently deleted regions of the SHIV genome to determine the intact provirus count; the second is a 2-long terminal repeat (2-LTR) DNA circle reaction to determine 2-LTR circle counts; and the third is a copy reference/DNA-shearing reaction targeting ribonuclease P/MRP subunit P30 (RPP30) to determine assay input cell equivalents and the DNA shearing index. All ddPCR reactions were performed using a Bio-Rad QX200 AutoDG ddPCR system with Bio-Rad ddPCR supermix for probes with no dUTP. After DNA shearing index correction and subtraction of intact 2-LTR circles, the intact proviral frequencies were reported per million input cells. The endpoint ddPCR data were collected using Bio-Rad QuantaSoft version 1.7.4.0917. Plasma virus genotypic analysis.[3] Total RNA was extracted from 50 μL plasma aliquots obtained from each viremic monkey using the MagMAX-96 Viral RNA Isolation Kit (Life Technologies) in conjunction with the Thermo Fisher Scientific KingFisher Flex automated extraction platform and eluted in 60 μL AVE buffer. The capsid coding area of gag in each sample was then individually amplified by RT-PCR using the SuperScript IV One-Step RT-PCR System (Life Technologies) and the Qiagen OneStep RT-PCR Kit according to the manufacturers’ recommended protocols. Amplification of the SHIV capsid coding region in each sample was performed using primers (SIV-CA-F [5′-CCAAAAACAAGTAGACCAACAG-3′] and SIV-CA-R [5′-TGCAAAAGGGATTGGCAC-3′]) and the products subjected to population-level bulk sequencing at Elim Biopharmaceuticals Inc. using the same primer set. To identify codon changes, capsid encoding sequences for each sample were aligned using DNA Sequencher Software (Gene Codes Corporation) with that of the parent challenge virus stock. A sequence alignment for major consensus HIV-1 subtype, HIV-2, and SHIV-SF162P3 capsid amino acid sequences was performed using BioEdit Sequence Alignment Editor version 7.2.6. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following subcutaneous administration, lenacapavir is slowly released but completely absorbed, with peak plasma concentrations occurring at 84 days post-dose. Absolute bioavailability following oral administration is low, approximately 6 to 10%. Tmax after oral administration is about four hours. The mean steady-state Cmax (%CV) is 97.2 (70.3) ng/ mL following oral and subcutaneous administration. According to population pharmacokinetics analysis, lenacapavir exposures (AUCtau, Cmax and Ctrough) were 29% to 84% higher in heavily treatment-experienced patients with an HIV-1 infection compared to subjects without an HIV-1 infection. A low-fat meal had negligible effects on drug absorption. Following a single intravenous dose of radiolabelled-lenacapavir in healthy subjects, 76% of the total radioactivity was recovered from feces and less than 1% from urine. Unchanged lenacapavir was the predominant moiety in plasma (69%) and feces (33%). The steady state volume of distribution was 976 L in heavily treatment-experienced patients with an HIV-1 infection. Lenacapavir clearance was 3.62 L/h in heavily treatment experienced patients with HIV-1 infection. Metabolism / Metabolites Metabolism played a lesser role in lenacapavir elimination. It undergoes CYP3A4- and UGT1A1-mediated oxidation, N-dealkylation, hydrogenation, amide hydrolysis, glucuronidation, hexose conjugation, pentose conjugation, and glutathione conjugation. The metabolites of lenacapavir have not been fully characterized. No single circulating metabolite accounted for >10% of plasma drug-related exposure. Biological Half-Life The median half-life ranged from 10 to 12 days following following oral administration, and 8 to 12 weeks following subcutaneous administration. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the prelicensure small, open-label clinical trial, serum aminotransferase elevations occurred in 10% of patients and were above 5 times the ULN in 2 (3%), both of whom also developed jaundice. In both instances, however, other causes of liver injury were identified, one case being attributed to alcohol related hepatitis and the other to “reconstitution syndrome” caused by the restoration of immune reactivity with the successful control of HIV replication. Both patients were continued on lenacapavir and recovered uneventfully. Since its approval and more widescale use, there have been no published reports of liver injury attributed to lenacapavir. One of the shortcomings of a long acting pharmaceutical agent such as lenacapavir is the inability to stop therapy promptly in the event of toxicity or intolerance. Finally, the restoration of immune reactivity as a result of adding lenacapavir to an antiretroviral regimen that is not fully effective can result in reconstitution syndrome and flares of chronic viral hepatitis in patients with preexisting chronic hepatitis B or C. Likelihood score: E (unlikely cause of idiosyncratic clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of lenacapavir during breastfeeding. Because the drug is greater than 98.5% protein bound, the amounts in milk are likely to be low. Achieving and maintaining viral suppression with antiretroviral therapy decreases breastfeeding transmission risk to less than 1%, but not zero. Individuals with HIV who are on antiretroviral therapy with a sustained undetectable viral load and who choose to breastfeed should be supported in this decision. If a viral load is not suppressed, banked pasteurized donor milk or formula is recommended. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding _In vitro_, lenacapavir is approximately 99.8% bound to plasma proteins. |
| 参考文献 | |
| 其他信息 |
Lenacapavir is a prescription medicine approved by the U.S. Food and Drug Administration (FDA). It is approved under two different brand names for the following uses:
Lenacapavir oral tablet and injection (brand name: Sunlenca) For the treatment of HIV in adults for whom other HIV medicines have not worked and who meet certain requirements, as determined by a health care provider. Lenacapavir for HIV treatment is always used in combination with other HIV medicines. Lenacapavir oral tablet and injection (brand name: Yeztugo) For HIV PrEP to reduce the risk of HIV in adults and adolescents who weigh at least 77 lb (35 kg), are HIV negative, and are at risk of getting HIV from sex. Lenacapavir for PrEP should always be used in combination with safer sex practices, such as using condoms, to reduce the risk of getting other sexually transmitted infections. Lenacapavir Sodium is the sodium salt form of lenacapavir, an inhibitor of human immunodeficiency virus type 1 (HIV-1) capsid function, with activity against HIV. Upon administration, lenacapavir targets and binds to the interface between HIV capsid protein (p24) subunits in hexamers. This inhibits capsid function, including capsid-mediated nuclear uptake of preintegration complexes, virion production and proper capsid core formation, thereby inhibiting HIV-1 replication. LENACAPAVIR SODIUM is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2022 and is indicated for hiv-1 infection and hiv infection. |
| 分子式 |
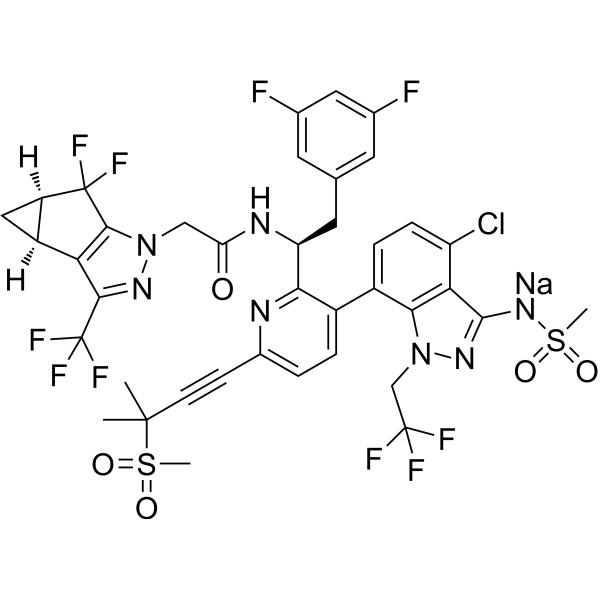
C39H31CLF10N7NAO5S2
|
|---|---|
| 分子量 |
990.26
|
| 精确质量 |
989.12546
|
| 元素分析 |
C, 47.30; H, 3.16; Cl, 3.58; F, 19.19; N, 9.90; Na, 2.32; O, 8.08; S, 6.48
|
| CAS号 |
2283356-12-5
|
| 相关CAS号 |
Lenacapavir;2189684-44-2; 2283356-18-1 (HCl); 2937414-47-4 (Lenacapavir pacfosacil); 2283356-12-5 (sodium)
|
| PubChem CID |
153435888
|
| 外观&性状 |
White to off-white solids at room temperature
|
| tPSA |
164 Ų
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
19
|
| 可旋转键数目(RBC) |
13
|
| 重原子数目 |
65
|
| 分子复杂度/Complexity |
2050
|
| 定义原子立体中心数目 |
3
|
| SMILES |
C(C1=NN(CC(=O)N[C@H](C2N=C(C#CC(C)(C)S(=O)(=O)C)C=CC=2C2C=CC(Cl)=C3C(NS(=O)(=O)C)=NN(CC(F)(F)F)C=23)CC2C=C(F)C=C(F)C=2)C2C(F)(F)[C@@H]3C[C@@H]3C1=2)(F)(F)F.[Na+]
|
| InChi Key |
SSXPGMNGIORJAQ-PZNXWHLTSA-M
|
| InChi Code |
InChI=1S/C39H32ClF10N7O5S2.Na/c1-36(2,63(3,59)60)10-9-21-5-6-22(23-7-8-26(40)30-32(23)57(17-37(43,44)45)54-35(30)55-64(4,61)62)31(51-21)27(13-18-11-19(41)14-20(42)12-18)52-28(58)16-56-34-29(33(53-56)39(48,49)50)24-15-25(24)38(34,46)47;/h5-8,11-12,14,24-25,27H,13,15-17H2,1-4H3,(H2,52,54,55,58);/q;+1/p-1/t24-,25+,27-;/m0./s1
|
| 化学名 |
sodium [4-chloro-7-[2-[(1S)-2-(3,5-difluorophenyl)-1-[[2-[(2S,4R)-5,5-difluoro-9-(trifluoromethyl)-7,8-diazatricyclo[4.3.0.02,4]nona-1(6),8-dien-7-yl]acetyl]amino]ethyl]-6-(3-methyl-3-methylsulfonylbut-1-ynyl)-3-pyridinyl]-1-(2,2,2-trifluoroethyl)indazol-3-yl]-methylsulfonylazanide
|
| 别名 |
GS-6207 sodium; Lenacapavir sodium; GS-HIV Sodium; 2283356-12-5; BDT58WJ9WE; UNII-BDT58WJ9WE;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.0098 mL | 5.0492 mL | 10.0984 mL | |
| 5 mM | 0.2020 mL | 1.0098 mL | 2.0197 mL | |
| 10 mM | 0.1010 mL | 0.5049 mL | 1.0098 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。