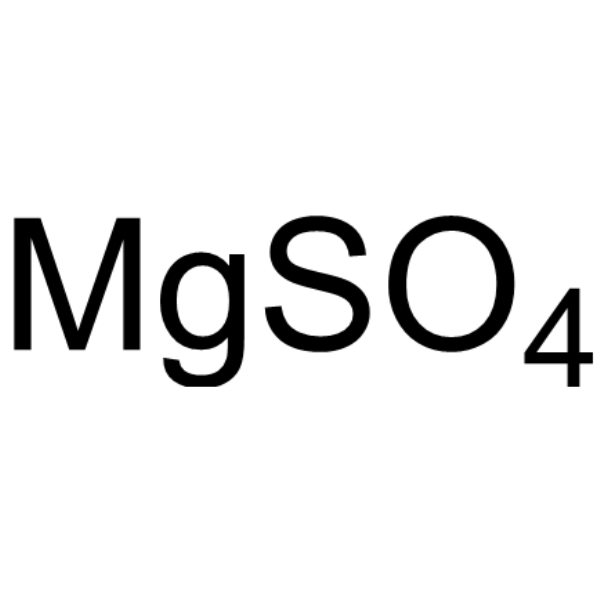
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50g |
|
||
| Other Sizes |
|

| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Magnesium is excreted solely by the kidney at a rate proportional to the serum concentration and glomerular filtration. Magnesium sulfate is excreted by the kidneys at a rate that varies from one patient to another but that is directly proportional to the serum concn and glomerular filtration. PLASMA CONCN OF MAGNESIUM INCR IN FETUS & APPROACH MATERNAL BLOOD VALUES AFTER MAGNESIUM SULFATE ADMIN IN ECLAMPSIA & PREECLAMPSIA. Metabolism / Metabolites None Magnesium is almost exclusively excreted in the urine, with 90% of the dose excreted during the first 24 hours after an intravenous infusion of MgSO4. The pharmacokinetic profile of MgSO4 after intravenous administration can be described by a 2-compartment model with a rapid distribution (a) phase, followed by a relative slow beta phase of elimination. Route of Elimination: Magnesium is excreted solely by the kidney at a rate proportional to the serum concentration and glomerular filtration. Half Life: 43.2 hours (for newborns) Biological Half-Life 43.2 hours (for newborns) |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Magnesium is the second most plentiful cation of the intracellular fluids. It is essential for the activity of many enzyme systems and plays an important role with regard to neurochemical transmission and muscular excitability. Magnesium sulfate reduces striated muscle contractions and blocks peripheral neuromuscular transmission by reducing acetylcholine release at the myoneural junction. Additionally, Magnesium inhibits Ca2+ influx through dihydropyridine-sensitive, voltage-dependent channels. This accounts for much of its relaxant action on vascular smooth muscle. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Intravenous magnesium increases milk magnesium concentrations only slightly and oral absorption of magnesium by the infant is poor, so maternal magnesium therapy is not expected to affect the breastfed infant's serum magnesium. Although intravenous magnesium sulfate given prior to delivery might affect the infant's ability to breastfeed, intention to breastfeed may be a more important determinant of breastfeeding initiation. Postpartum use of intravenous magnesium sulfate for longer than 6 hours appears to delay the onset of lactation. One group of experts recommends reserving postpartum magnesium sulfate prophylaxis for those women with persistent neurologic symptoms within 7 days of birth. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk One mother who received intravenous magnesium sulfate for 3 days for pregnancy-induced hypertension had lactogenesis II delayed until day 10 postpartum. No other specific cause was found for the delay, although a complete work-up was not done. A subsequent controlled clinical trial found no evidence of delayed lactation in mothers who received intravenous magnesium sulfate therapy. Some, but not all, studies have found a trend toward increased time to the first feeding or decreased sucking in infants of mothers treated with intravenous magnesium sulfate during labor because of placental transfer of magnesium to the fetus. Another study found that among women with severe pre-eclampsia who received intravenous magnesium sulfate for up to one day postpartum and who intended to breastfeed, 85% of infants receiving routine well-baby care and 69% of those admitted to the NICU, breastfeeding was successfully initiated. A study randomized women with preeclampsia to receive intravenous magnesium sulfate for either 6 or 24 hours postpartum. There was no difference in the rate of eclampsia between the two groups. However, those who received the infusion for 24 hours had a delayed onset of lactation, 36.5 hours compared with 25.7 hours in the 6-hour group. A prospective, multicenter, randomized, controlled trial in 9 Latin American maternity hospitals compared patients with severe pre-eclampsia who had received at least 8 grams of magnesium sulfate prior to placebo. Patients were randomized to continue magnesium sulfate for 24 hours postpartum (n = 555) or stopping the infusion (n = 558). The time to lactation was significantly delayed in those who received magnesium sulfate postpartum (24.1 vs. 17.1 hours). A study randomized pregnant women with moderate to severe pre-eclampsia to receive magnesium sulfate intravenously infused at the same dose (not specified) for 8 or 24 hours. In patients who received the 8-hour infusion, the mean time to initiate breastfeeding was 14.6 hours compared to 24.3 hours in the patients who received the 24-hour infusion, which was a statistically significant difference. A retrospective chart review of mothers who delivered at the University of Chicago found that intravenous magnesium sulfate during delivery was associated with over 60% reduced odds of breastfeeding initiation compared to mothers who received no magnesium. Protein Binding 25-30% Toxicity Data LD50: 1200 mg/kg (rat, parenteral-subcutaneous). The first warning of impending toxicity is loss of the patellar reflex at plasma concentrations between 3.5 and 5 mmol/L. Respiratory paralysis occurs at 5 to 6.5 mmol/L. Cardiac conduction is altered at greater than 7.5 mmol/L, and cardiac arrest can be expected when concentrations of magnesium exceed 12.5 mmol/L. Interactions ADMIN OF MAGNESIUM SULFATE IN PREECLAMPSIA & ECLAMPSIA POTENTIATES NEUROMUSCULAR BLOCKADE PRODUCED BY D-TUBOCURARINE, DECAMETHONIUM, & SUCCINYLCHOLINE. When barbiturates, opiates, general anesthetics, or other CNS depressants are administered concomitantly with magnesium sulfate, dosage of these agents must be carefully adjusted because of the additive central depressant effects. ...Magnesium inhibited extracellular calcium entry, intracellular calcium release, cytosolic calcium oscillations, and phasic contractions of myometrial smooth muscle /induced by oxytocin and other uterotonic agonists/. ...The present in vivo rat study examined the effect of magnesium sulfate to alter the pressor response to norepinephrine (NE) and angiotensin II (A II). Magnesium doses were chosen to approximate those used in treating preeclampsia. NE resulted in a significant rise in mean arterial pressure (delta MAP, 46 +/- 3.7 mmHg; p<0.001). A II also resulted in a significant rise in MAP (delta MAP, 23 +/- 3.6 mmHg, p<0.02). Magnesium sulfate alone had no significant effect on MAP but attenuated the pressor response to both NE (delta MAP, 16 +/- 1.5 mmHg) and A II (delta MAP, 12 +/- 2.5 mmHg). After discontinuation of the magnesium sulfate infusion, the control pressor responses to NE and A II were again seen (delta MAP, 39 +/- 3.5 mmHg and delta MAP, 28 +/- 4.2 mmHg, respectively). Although magnesium sulfate is not a primary antihypertensive agent, it may have effects on blood pressure by attenuating the actions of circulating vasoconstrictors. For more Interactions (Complete) data for MAGNESIUM SULFATE (6 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse subcutaneous 645 mg/kg LD50 Rat subcutaneous 1200 mg/kg |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Analgesics; Anesthetics; Anti-Arrhythmia Agents; Anticonvulsants; Calcium Channel Blockers; Cathartics; Tocolytic Agents AN EFFECTIVE & WIDELY EMPLOYED SALINE CATHARTIC. /HEPTAHYDRATE, USP/ FULL DOSES OF SALINE CATHARTICS (15 G MAGNESIUM SULFATE OR ITS EQUIVALENT) PRODUCE A SEMIFLUID OR WATERY EVACUATION IN 3 HR OR LESS. LOW DOSES PRODUCE A LAXATIVE EFFECT WITH GREATER LATENCY. A COLD, WET COMPRESS OF MAGNESIUM SULFATE SOLN IN WATER HAS BEEN EMPLOYED IN TREATMENT OF SUCH SKIN DISORDERS AS ERYSIPELAS. HOT CONCN AQ SOLN...(ABOUT 1 LB/PINT OF WATER) ARE SOMETIMES USED IN TREATMENT OF DEEP-SEATED INFECTIONS, CLOTHS BEING SATURATED & APPLIED WHILE HOT. ACTION MUCH LIKE THAT OF POULTICE. /HEPTAHYDRATE/ For more Therapeutic Uses (Complete) data for MAGNESIUM SULFATE (32 total), please visit the HSDB record page. Drug Warnings SOME ABSORPTION OF COMPONENT IONS OF SALINE CATHARTICS DOES OCCUR, & IN CERTAIN INSTANCES THEY MAY PRODUCE SYSTEMIC TOXICITY. IN AN INDIVIDUAL WITH IMPAIRED RENAL FUNCTION, ACCUM OF MAGNESIUM IONS IN BODY FLUIDS MAY BE SUFFICIENT TO CAUSE INTOXICATION. MAGNESIUM CATHARTICS SHOULD BE ADMIN ONLY IF RENAL FUNCTION IS ADEQUATE. THE DRUG IS GENERALLY SAFE BUT CAN CAUSE TEMPORARY LOSS OF DEEP TENDON REFLEXES IN MOTHER & MAY SUPPRESS SKELETAL MUSCLE ACTIVITY IN NEONATE. IT SHOULD NOT BE USED IN PT WITH HEART DISEASE... NEONATE MAY BE DROWSY & EXHIBIT RESP DIFFICULTIES & DIMINISHED MUSCLE TONE. HOWEVER...NO RELATIONSHIP BETWEEN PLASMA MAGNESIUM CONCN OF BLOOD COLLECTED FROM UMBILICAL CORD & THE APGAR SCORE /HAS BEEN FOUND/. Patients receiving parenteral magnesium sulfate shold be observed carefully, and serum magnesium concn should be monitored to avoid overdosage. ... An IV preparation of a calcium salt (e.g., calcium gluconate) should be readily available for use when magnesium sulfate is given IV. /Magnesium sulfate injection/ For more Drug Warnings (Complete) data for MAGNESIUM SULFATE (14 total), please visit the HSDB record page. Pharmacodynamics Magnesium sulfate is a small colorless crystal used as an anticonvulsant, a cathartic, and an electrolyte replenisher in the treatment of pre-eclampsia and eclampsia. It causes direct inhibition of action potentials in myometrial muscle cells. Excitation and contraction are uncoupled, which decreases the frequency and force of contractions. Magnesium sulfate is gaining popularity as an initial treatment in the management of various dysrhythmias, particularly torsades de pointes, and dyrhythmias secondary to TCA overdose or digitalis toxicity. |
| 分子式 |
MGO4S
|
|---|---|
| 分子量 |
120.3676
|
| 精确质量 |
119.936
|
| CAS号 |
7487-88-9
|
| 相关CAS号 |
10034-99-8 (heptahydrate)
|
| PubChem CID |
24083
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.07 g/mL at 20 °C
|
| 沸点 |
330ºC at 760 mmHg
|
| 熔点 |
1124 °C
|
| 蒸汽压 |
<0.1 mm Hg ( 20 °C)
|
| tPSA |
88.64
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
6
|
| 分子复杂度/Complexity |
62.2
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
CSNNHWWHGAXBCP-UHFFFAOYSA-L
|
| InChi Code |
InChI=1S/Mg.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)/q+2;/p-2
|
| 化学名 |
magnesium;sulfate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O: 25 mg/mL
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 8.3077 mL | 41.5386 mL | 83.0772 mL | |
| 5 mM | 1.6615 mL | 8.3077 mL | 16.6154 mL | |
| 10 mM | 0.8308 mL | 4.1539 mL | 8.3077 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Evaluation of the Role of Magnesium in Prevention of AF Post Cardiac Surgery
CTID: NCT06675500
Phase: Phase 3 Status: Not yet recruiting
Date: 2024-11-05