| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|

| 靶点 |
NMDA Receptor
|
|---|---|
| 体外研究 (In Vitro) |
先前对Lanicemine和氯胺酮的研究表明,这两种化合物与NMDA通道孔内的位点结合具有低至中等亲和力,表现出很强的电压依赖性,并且缺乏NR2A和NR2B亚基选择性(表1)。然而,在稳态浓度下,氯胺酮在谷氨酸去除和重新施用后更容易被捕获在NMDA通道孔内(氯胺酮捕获率为86%,而Lanicemine的氯胺酮捕获率为54%)。在正常的锥体细胞驱动的突触传递条件下,低捕获理论上保留了使用依赖的通道块。因此,虽然NMDARs在中枢神经系统中普遍表达,但Lanicemine的低捕获特性可能会导致通道阻滞到大脑的那些元素,如皮质中间神经元,具有高水平的强亢活性。由于皮质中间神经元上NMDAR活性的选择性减少已被证明会增加自发性,因此高频(γ波段~ 40 Hz)脑电图(γ波段脑电图)可以作为NMDA通道阻滞剂,特别是Lanicemine的有用生物标志物。[1]
|
| 体内研究 (In Vivo) |
最小的拟精神病副作用与兰司明的长期抗抑郁功效相关[1]。除了激活负责产生伽马脑电图 (EEG) 的大脑回路外,Lanicemine(3、10 或 30 mg/kg;腹腔注射)还可以独立于通常与氯胺酮相关的更普遍的系统级干扰来修改这些网络。[1]。
通过定量脑电图(qEEG)客观调整低捕获NMDA通道阻滞剂AZD6765(拉尼西明)与氯胺酮的剂量匹配,我们证明了NMDA通道阻滞剂在产生抗抑郁疗效的同时,可不引发拟精神病和分离性副作用。此外,通过安慰剂对照数据,我们表明NMDA通道阻滞剂的抗抑郁反应可通过重复间歇给药得以维持。这些数据共同为开发基于谷氨酸能机制的新型难治性情绪障碍疗法提供了路径。[1] 拉尼西明相对于氯胺酮在人体的EEG、生理及解离效应[1] 为验证氯胺酮与拉尼西明在临床前研究中观察到的差异化EEG/副作用特征是否适用于人类,我们在健康志愿者中开展了qEEG交叉研究。23名随机受试者中,14人接受75mg拉尼西明治疗,19人接受150mg拉尼西明,17人接受氯胺酮,15人接受安慰剂。研究因氯胺酮输注期间发生两起严重不良事件(体位性低血压所致晕厥)而提前终止。输注结束时,氯胺酮与拉尼西明均引起γ波段EEG显著增强,且150mg拉尼西明的基线校正γ-EEG与氯胺酮(0.5mg/kg)在统计学上无差异(图2左及右上)。此外,氯胺酮与150mg拉尼西明均显著降低前额叶θ-协调性(一种推定与早期抗抑郁治疗反应相关的EEG生物标志物)(图2左)。拉尼西明组未出现严重不良事件。 Lanicemine/拉尼西明单次剂量(100mg)在难治性重度抑郁症(MDD)患者中的探索性安全性与疗效试验[1] 转化研究与前期临床前数据28表明,拉尼西明在75-150mg剂量范围内存在不引发拟精神病症状的治疗窗口。因此我们开展先导研究,评估该剂量范围(100mg)是否具备良好耐受性同时仍能产生抗抑郁信号。在IIA期单药治疗研究(研究1)中,34名难治性患者(平均HAM-D-17评分∼25;附表1)随机接受单次静脉输注拉尼西明100mg(n=16,男7/女9)或安慰剂(n=18,男7/女11)。 拉尼西明/Lanicemine在中重度MDD且抗抑郁药治疗史不佳患者中的附加多剂量输注疗效试验[1] 基于研究1的探索性数据,我们设计了第二项II期研究(研究9),旨在评估3周内每周三次重复给药方案能否巩固并扩展单次给药观察到的治疗获益。在研究9中,我们考察了在现有抗抑郁治疗基础上联合拉尼西明重复给药对中重度MDD门诊患者(既往对多种抗抑郁药反应不佳)的症状改善作用。输注治疗3周后停止,并在后续5周未给药观察期内评估抗抑郁效应的持续性。 |
| 动物实验 |
Animal/Disease Models: Male SD (SD (Sprague-Dawley)) rat [1]
Doses: 3, 10 or 30 mg/kg Route of Administration: intraperitoneal Experimental Results: Spontaneous gamma-band EEG produced significant dose-dependent elevations, but only with ketamine Gamma changes are closely associated with increases in locomotor activity. Effects of ketamine and Lanicemine on EEG in rodent models [1] Male Sprague-Dawley rats (n=6–9) were implanted with frontal and temporal skull screw electrodes for continuous EEG recording and trained to perform a single-tone operant discrimination task for food reward. EEG was recorded and behavioral performance was evaluated for a 30-min period before dosing and for three 30-min periods following dosing with intraperitoneal lanicemine (3, 10 or 30 mg kg−1), ketamine (1, 3, 10 or 30 mg kg−1) or vehicle control. EEG data acquired by Neuralynx were imported to NeuroExplorer Ver. 3.183 software suite. Consecutive 10-s epochs of EEG data from each channel were subjected to a fast Fourier transform, from which EEG power density was computed from 1 to 50 Hz. Lanicemine studies in human [1] All studies in man were approved by the institutional review boards at each site and were conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Conference on Harmonization guideline E6: Good Clinical Practice. All participants provided written, informed consent before study entry and had the right to withdraw from the study at any time. EEG, physiological and dissociative effects of Lanicemine relative to ketamine in human (phase I, D2285M00008/NCT01130909) [1] A phase I, randomized, double-blind, four-way, crossover study in healthy subjects was performed at a single center in France between May 2010 and January 2011 (D2285M00008/NCT01130909). Males aged 30–45 years, with body mass index 18–30 kg m−2 and non-smoking status for at least 4 weeks, without clinically relevant acute or chronic disease, received lanicemine 75 mg, lanicemine 150 mg, ketamine 0.5 mg kg−1 or placebo as single intravenous (i.v.) administrations. Washout was ⩾7 days between study periods. Single-dose (100 mg) exploratory safety and efficacy trial of Lanicemine in patients with treatment-resistant MDD (phase IIA, D6702C00001/NCT00491686) [1] The phase IIA, double-blind, randomized study (D6702C00001/NCT00491686; study 1) was performed at five centers in the United States between July 2007 and November 2007. It consisted of a screening period (⩽30 days), one inpatient treatment period, and one follow-up visit 7–10 days after treatment. Outpatients (men and women) aged 21–65 years with DSM-IV-TR-diagnosed MDD, confirmed by the MINI, a history of poor response to ⩾2 antidepressants, and baseline Hamilton Rating Scale for Depression (HAM-D-17) score ⩾20 were eligible. Exclusion criteria included: current episode of depression ⩽12 weeks or ⩾5 years; history of DSM-IV Axis I disorder other than MDD or substantial Axis II disorder; use of mood stabilizers, other antipsychotic or psychoactive drugs within 7 days of day 1 or fluoxetine or monoamine oxidase inhibitors within 14 days of day 1 of the treatment period; and evidence of other clinically relevant disease. Lanicemine 100 mg or placebo (0.9% saline) was administered as single i.v. infusions (30 ml volume over 60 min). The primary efficacy evaluation was change in Montgomery-Åsberg Depression Rating Scale (MADRS) total score from baseline to 24 h post infusion. Secondary variables included: change in MADRS total score at other scheduled time points; Bond-Lader Visual Analogue Scale; Brief Psychiatric Rating Scale; and CogState (CogState, Melbourne, Australia). Safety evaluations included: adverse events, vital signs, physical examination, clinical laboratory evaluations and electrocardiograms. Adjunctive, multiple-infusion efficacy trial of Lanicemine in patients with moderate-to-severe MDD and a history of poor response to antidepressants (phase IIB, D6702C00009/NCT00781742) [1] The phase IIB, double-blind, randomized, outpatient study (D6702C00009/NCT00781742; study 9) was performed at 30 centers in the United States between October 2008 and March 2010. It consisted of a screening period (⩽30 days), a 3-day placebo run-in (when patients received one single-blind placebo infusion (0.9% saline)), and a 3-week treatment period, followed by a 5-week treatment-free follow-up. Patients were randomized in a 1:1:1 ratio to Lanicemine 100 mg, lanicemine 150 mg or placebo (three i.v. infusions per week) as adjunct to ongoing psychotropics that included at least one antidepressant. The predefined primary efficacy variable was change from randomization to week 3 in MADRS total score. Secondary variables included: MADRS score change at other scheduled assessments; remission (that is, MADRS score ⩽10); response (that is, ⩾50% reduction from baseline in MADRS score); Hamilton Rating Scale for Anxiety (HAM-A; anxiety); HAM-D-17 and QIDS-SR-16 (depressive symptoms); CGI-S and Clinical Global Impression of Improvement (CGI-I; global improvement); and Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q; quality of life). Efficacy evaluations were performed at weekly intervals from baseline (randomization) to week 8. Changes in QIDS-SR-16 score at day 1 and MADRS score at day 3 were also measured to assess onset of effect. Change from baseline in MADRS total score and continuous secondary efficacy variables were compared between the two Laniceminegroups and placebo at week 3 with LOCF in the ITT analysis set, using an analysis of covariance model with baseline MADRS total score as a covariate, with treatment, MDD disease severity and comorbid generalized anxiety disorder status as fixed effects, and pooled center as a random effect. A logistic regression model including treatment and baseline in the model was used for categorical secondary efficacy variables. |
| 毒性/毒理 (Toxicokinetics/TK) |
Single-dose (100 mg) exploratory safety and efficacy trial of Lanicemine in patients with treatment-resistant MDD [1]
Translational and previous preclinical data suggested a psychotomimetic-free therapeutic window for lanicemine in humans at doses of 75–150 mg. We therefore conducted a pilot study to determine whether a dose in this range (100 mg) might be relatively well tolerated and yet still provide an antidepressant signal. In a phase IIA monotherapy study (study 1), 34 treatment-resistant patients (mean HAM-D-17 score ∼25; Supplementary Table 1) were randomized to a single infusion of lanicemine 100 mg i.v. (n=16 (7, male; 9, female)) or placebo (n=18 (7, male; 11, female)). Lanicemine 100 mg was generally well tolerated, with the most common adverse event being dizziness (Supplementary Table 2). Lanicemine produced no clinically meaningful effects on psychotomimetic symptoms measured by the Brief Psychiatric Rating Scale (mean±s.e. at 1 h: 22.8±1.1 for lanicemine vs 23.9±1.2 for placebo; at 4 h: 23.1±1.2 vs 24.4±1.5), dissociative symptoms measured by the CADSS (least squares mean (LSM)±s.e. change from baseline at 1 h: 0.6 (0.59) for lanicemine vs −0.8 (0.55) for placebo), or cognitive functions measured by CogState (Supplementary Figures 1 and 2). There were no serious adverse events reported during treatment. At the 24-h time point, we failed to observe a statistically significant difference in the change in MADRS scores between Lanicemine vs placebo. However, this comparison was confounded by a strong placebo effect (for example, 14.2 MADRS score change) at this time point, rendering the meaningfulness of the non-statistically significant, numerically greater change for Lanicemine vs placebo (2.44, P=0.472) difficult to interpret. However, we observed statistically significant differences (according to prespecified criteria for this exploratory study) for lanicemine vs placebo in MADRS scores at 1 and 72 h after the infusion (P=0.183 and 0.089, respectively) (Supplementary Figure 3). An antidepressant-like effect was also indicated by changes in sad/happy Bond-Lader Visual Analogue Scale at 4 h (P=0.137). The trend for the antidepressant effect of lanicemine peaked at 72 h (MADRS score change vs placebo of −5.7 (P=0.089)) and had dissipated vs placebo by 10–13 days after the single i.v. infusion, while remaining on average 10 points below baseline measures. Safety evaluations included safety and tolerability assessments, adverse events, pupil size and electronystagmography, and subjective dissociative effects measured by the 27-item Clinician Administered Dissociative States Scale (CADSS). Opticokinetic parameters were measured 25 min after start of infusion using Metrovision MON 2008H and CADSS was assessed at prespecified times up to 8 h after start of infusion. |
| 参考文献 | |
| 其他信息 |
Lanicemine has been used in trials studying the treatment and basic science of Depression, Major Depressive Disorder, and Treatment Resistant Major Depressive Disorder.
There is a rapidly growing interest in the development of glutamatergic drugs, especially NMDAR antagonists, for the treatment of severe mood disorders.5 Here, we report robust and sustained antidepressant effects for a low-trapping NMDA channel blocker, Lanicemine (100 mg), at doses without the limiting prominent dissociative side effects observed with ketamine. Completed studies with lanicemine now encompass the largest pool of depressed patients (n>120) exposed to an NMDA channel blocker to date, and represent a stringent test of the hypothesis that NMDAR antagonists can deliver antidepressant efficacy independent of psychotomimetic side effects. Second, while study 1 and published data by Zarate et al provide evidence supporting a single-dose antidepressant effect for Lanicemine, a similar single-dose trend was not as robustly observed in study 9. Given that study 9 (in contrast to study 1) was an adjunctive study and most reports to date with ketamine have also been monotherapy studies, an open question remains regarding the extent to which concomitant medications (that is, benzodiazepine administration) may alter the time course of antidepressant treatment effects for NMDA channel blockers. Preliminary reports on ketamine when used adjunctively with antidepressants also suggest a delayed onset of efficacy. Finally, while study 9 provides limited data regarding the efficacy and safety of repeated intermittent administrations, it will be critical to better characterize and understand the long-term safety and efficacy profile of NMDA channel blockers including lanicemine. These questions and others are currently being addressed in ongoing studies (for example, Study 31: ClinicalTrials.gov Identifier: NCT01482221). Lanicemine, a low-trapping NMDA channel blocker, demonstrated antidepressant effects in patient studies, with fewer dissociative and psychotomimetic symptoms than ketamine at dose exposures that caused similar changes in cortical activation. In clinical studies, lanicemine produced robust and significant efficacy without clinically appreciable dissociative and psychotomimetic adverse effects. These data are consistent with the pharmacological separation of efficacy from psychotomimetic side effects observed in preclinical and phase I studies. Importantly, in a 3-week, placebo-controlled phase IIB study of patients with moderate-to-severe MDD, repeated administration of lanicemine (100 or 150 mg per infusion) at 3-day intervals provided sustained antidepressant efficacy, without psychotomimetic effects. The results of these studies demonstrate that an NMDA channel blocker can achieve antidepressant responses in the absence of prominent psychotomimetic effects and are sustained with repeated dosing. The putative antidepressant characteristics of lanicemine are being explored in ongoing clinical trials. [1] |
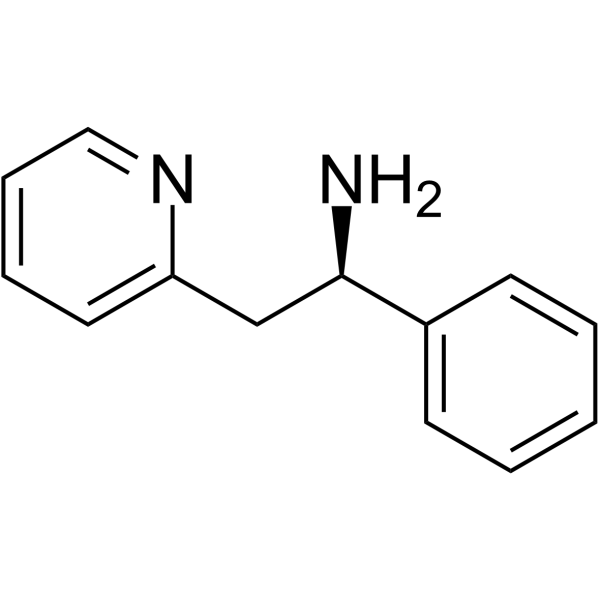
| 分子式 |
C13H14N2
|
|---|---|
| 分子量 |
198.26
|
| 精确质量 |
198.115
|
| 元素分析 |
C, 78.75; H, 7.12; N, 14.13
|
| CAS号 |
190581-71-6
|
| 相关CAS号 |
(Rac)-Lanicemine;61890-25-3;Lanicemine;153322-05-5
|
| PubChem CID |
10198092
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
1.7
|
| tPSA |
38.9
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
15
|
| 分子复杂度/Complexity |
175
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C1=CC=C(C=C1)[C@@H](CC2=CC=CC=N2)N
|
| InChi Key |
FWUQWDCOOWEXRY-CYBMUJFWSA-N
|
| InChi Code |
InChI=1S/C13H14N2/c14-13(11-6-2-1-3-7-11)10-12-8-4-5-9-15-12/h1-9,13H,10,14H2/t13-/m1/s1
|
| 化学名 |
(1R)-1-phenyl-2-pyridin-2-ylethanamine
|
| 别名 |
(R)-Lanicemine; 190581-71-6; CHEMBL466285; SCHEMBL13557819; (1R)-1-phenyl-2-pyridin-2-ylethanamine; (1R)-1-phenyl-2-(pyridin-2-yl)ethan-1-amine; .
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.0439 mL | 25.2194 mL | 50.4388 mL | |
| 5 mM | 1.0088 mL | 5.0439 mL | 10.0878 mL | |
| 10 mM | 0.5044 mL | 2.5219 mL | 5.0439 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。