| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Microbial Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
对于 HT-29、MCF-7、A375、K562 和 NCM460 细胞系,Cyclo(Pro-Leu)(化合物 7)(1.23 μg/mL-300 μg/mL;48 h)表现出细胞毒性作用,IC50 值为 101.56分别为μM、78.78μM、51.13μM、21.72μM和775.86μM[3]。
根据物理化学方法,纯化的抑制物质被鉴定为环(l-Leu-l-Pro)。通过尖端培养法测定,寄生曲霉SYS-4(=NRRL2999)产生黄曲霉毒素的50%抑制浓度为0.20 mg ml(-1)。高浓度(超过6.0 mg ml(-1))的环(l-Leu-l-Pro)进一步抑制了真菌的生长。环(D-亮氨酰基-D-脯氨酰基)和环(L-缬氨酸-L-脯氨酰)也观察到类似的抑制活性,而环(D-脯氨酰基-L-亮氨酰基)与环(L-脯氨酰-D-亮氨酰)的抑制活性较弱。逆转录PCR分析表明,环(L-亮氨酸-L-脯氨酰)抑制黄曲霉毒素相关基因aflR、hexB、pksL1和dmtA的转录。这是首次报道一种影响黄曲霉毒素产生的环二肽。[1] 抑制活性的特征。[1] 我们使用微量滴定琼脂平板法研究了市售的环(l-Leu-l-Pro)对寄生A.NFRI-95突变体NA积累的抑制活性(表1)。发现这种浓度大于3.5 mg ml-1的物质可以完全抑制a.parasiticus NFRI-95对NA的积累。在1.0 mg ml-1的浓度下,它也部分抑制了NA的积累,这表明该浓度可能接近50%的抑制浓度。Cyclo(d-Leu-d-Pro)是Cyclo(l-Leu-l-Pro)的立体异构体,其活性与Cyclo(l-Leu-l-Prro)相似(图1D)。l-亮氨酸、d-亮氨酸、l-脯氨酸或d-脯氨酸或这些氨基酸的组合没有显示出任何抑制活性,表明这种抑制作用是针对环二肽的。还检测了其他四种含有环(l-Leu-l-Pro)氨基酸之一的环二肽(表1)。Cyclo(l-Leu-l-Gly)在浓度达到3.5 mg ml−1之前没有表现出任何活性,在6.0 mg ml−着1的浓度下,它对真菌的NA产生有微弱的抑制作用。另外两种环二肽,环(l-Gly-l-Pro)和环(d-Ala-l-Pro。相比之下,即使在0.3 mg ml-1的浓度下,环(l-Pro-l-Val)也能抑制NA的积累,这低于环(Leu-Pro)显示出抑制作用的浓度。这些结果表明,由一个疏水性氨基酸和脯氨酸组成的环二肽结构可能有助于抑制活性 我们更详细地比较了四种异构体的影响,即环(l-Leu-l-Pro)、环(d-Leu-d-Pro)、环(l-亮氨酸-d-Pro)和环(d-亮氨酸-l-Pro)(图1D和表1)cyclo(l-Leu-l-Pro)在3.5 mg ml-1的浓度下完全抑制了寄生A.NFRI-95对NA的积累,也抑制了孢子的形成。Cyclo(d-Leu-d-Pro)在相同浓度下也显示出对NA积累的完全抑制作用,而对孢子形成的影响似乎较弱,因为即使在6 mg ml-1的浓度下,真菌也能产生孢子。相比之下,其他两种异构体,环(l-Leu-d-Pro)和环(d-Leu-l-Pro)的活性要低得多。Cyclo(l-Leu-d-Pro)在12.0 mg ml−1的浓度下可以完全抑制NA积累,而Cyclo(d-Leu-l-Pro)即使在最高浓度下也不能完全抑制NA的积累(表1和图1D)。有趣的是,这些异构体影响了孢子的颜色,更高的浓度使孢子颜色从浅绿色变为深绿色。。[1] cyclo(l-Leu-l-Pro)对黄曲霉毒素产生的影响。[1] A.寄生虫SYS-4(=NRRL2999)在尖端培养物中与不同浓度的环(l-Leu-l-Pro)或环(d-Leu-d-Pro)一起孵育。通过这种方法确定,浓度为3.5 mg ml−1的任何一种抑制剂都完全抑制了黄曲霉毒素的产生(图4A,泳道2和3),而在这种浓度下,真菌的生长只受到轻微影响。相比之下,当这两种物质的浓度均为6 mg ml−1时,菌丝体重量显著降低,表明高浓度的这些物质也可以抑制真菌生长。添加这些物质不会改变培养基的pH值。TLC还表明,菌丝体中没有积累任何与黄曲霉毒素产生途径相关的中间体(图4A,泳道5和6) 在尖端培养中,这两种物质的黄曲霉毒素产量在0至1.0 mg ml−1的浓度范围内都有所下降(图4B)。环(l-Leu-l-Pro)和环(d-Leu-d-Pro)的50%抑制浓度几乎相同,分别为0.20和0.13 mg ml−1。相比之下,在这些浓度下,真菌的生长没有受到影响,这表明这些环二肽特异性地抑制了黄曲霉毒素的产生。然而,较高浓度(超过6 mg ml-1)的物质抑制了真菌的生长(图4B)。 抑制黄曲霉毒素相关基因的转录。[1] 通过RT-PCR分析了环(l-Leu-l-Pro)对转录的影响。A.寄生菌SYS-4通过尖端培养法在含有或不含有浓度为3.5 mg ml−1的环(l-Leu-l-Pro)的YES培养基中培养。在没有抑制剂的情况下检测到黄曲霉毒素相关基因aflR、hexB、dmtA和pks的表达(图5A)。然而,这些基因的表达在3.5 mg ml-1的浓度下被环(l-Leu-l-Pro)完全抑制,而真菌生长在该浓度下仅受到轻微影响。培养滤液的薄层色谱也证实,抑制剂在3.5 mg ml−1的浓度下完全抑制了黄曲霉毒素的产生(图5B)。cmd基因是一种组成型表达的钙调素基因,与黄曲霉毒素的产生无关,在环(l-Leu-l-Pro)存在的情况下,cmd基因的表达没有受到抑制(图5A)。 我们还研究了环(l-Leu-l-Pro)对酶活性的影响。在饲养实验中,当将杂色曲霉素添加到培养基中时,200μl培养基中产生了56.4±5.7μg的黄曲霉毒素。相比之下,添加2.0 mgcyclo(l-Leu-l-Pro)ml−1会导致黄曲霉毒素产量急剧下降,降至无抑制剂时的7.1%(培养基中黄曲霉毒素总量为4.0±6.4μg)。杂色曲霉素产生黄曲霉毒素的抑制可能是由于环(l-Leu-l-Pro)抑制了酶基因的表达。 |
| 体内研究 (In Vivo) |
内生链霉菌菌株是新型生物活性分子的潜在来源。在这项研究中,cyclo(l-Leu-l-Pro) or gancidin W(GW)是从椭圆海岸树树皮中获得的内生放线菌属链霉菌SUK10中分离出来的,并对其进行了体内抗伯氏疟原虫PZZ1/100的测试<使用雄性ICR品系小鼠的4天抑制试验方法,在6.25和3.125μg kg-1体重下,strong>cyclo(l-Leu-l-Pro)或gancidin W/GW在第四天的抑制率接近80%。分别以盐酸奎宁和生理盐水作为阳性和阴性对照,比较两种浓度的cyclo(l-Leu-l-Pro)或gancidin W/GW,用3.125μg kg-1体重治疗的小鼠中有50%在感染后存活了11个月以上,几乎达到了正常小鼠的寿命。用环(l-Leu-l-Pro)或gancidin W/GW处理的小鼠血液样本中选定酶和蛋白质的生化测试也在正常水平内;此外,内部重要器官未发现异常或损伤。这些发现表明,这种从链霉菌SUK10中分离出来的生物活性化合物具有非常低的毒性,是动物模型中潜在的抗疟疾药物的良好候选者。[2]
GW/cyclo(l-Leu-l-Pro)或gancidin W在五种不同浓度的窄范围内进行抗疟疾筛查,以确定哪种剂量具有最佳的抗疟疾活性。寄生虫血症密度值直接反映了抑制百分比值,其中抑制百分比越高,治疗越有效。发现3.125μg kg−1 bw剂量的抑制率最大。3.125μg kg−1 bw组与其他四种浓度组之间的抑制率接近80%,存在显著差异(P<0.05,n=6)。6.25μg kg−1 bw剂量下的抑制率也高于65%,这被认为是体内抗疟疾活性的基准,这一值远优于其他三种剂量浓度(表3)。[2] 用3.125μg kg−1 bw剂量的<强>环(l-Leu-l-Pro)或gancidin W/GW治疗的小鼠的存活时间几乎是12.5μg kg-1 bw剂量小鼠的两倍,并且按比例高于6.25μg kg-1 bw的小鼠(图3)。如前所述,体内和体外分析都预测,在较高的寄生虫抑制率下,接受治疗的小鼠的存活时间会更长。以3.125μg kg−1 bw的剂量浓度用cyclo(l-Leu-l-Pro)或gancidin W/GW治疗,小鼠存活时间最长(235.53±2.20天),明显高于其他剂量浓度(P<0.05,n=6)。与此同时,令人惊讶的是,用该浓度的cyclo(l-Leu-l-Pro)或gancidin W/GW单独治疗的组中剩余的50%(n=3)存活至感染后291.13±0.5天。根据之前的文献,这段存活时间被认为几乎达到了正常雄性小鼠在大约12-18个月时的寿命。直到感染后411天,所有PC小鼠都存活了下来,这一观察结果与之前的体内抗疟疾研究结果一致,而NC小鼠在感染后存活了7-9天,这也记录在本研究中。[2] |
| 酶活实验 |
RT-PCR。[1]
A.寄生菌SYS-4(=NRRL2999)在YES肉汤或补充了3.5 mg环(l-Leu-l-Pro)ml−1的YES肉汤中培养。菌丝体培养63小时后,使用FastPrep FP100A(Q-BIO基因;BIO 101)用TRI试剂破坏菌丝体。根据制造商的说明制备总RNA,然后用无RNase的DNase处理。使用所得总RNA和RT-PCR试剂盒进行逆转录(RT)-PCR。使用的引物是aflR-BamHI-F(CGCGGATCCATGTGTGACATATCTCCC)和aflR-HindIII-R(CCCCAAGCTTCATTCGATGCAGGTAATC)用于aflR(登录号AF441437),HexB-F1(CTGCGGGTGGAGCTGCA)和HexB-R1(CAAGCTCCAAGGGCGGGGGGGC)用于己酸合酶基因(登录号AF 391094),pKSL1-F1(CCAGACAGCCCTATCTAG)和pKSL1-R1(GGAGTCCAGGTGATTCAGC)用于聚酮合成酶基因pKSL1(登录号L42761)6),用于O-甲基转移酶I基因的MT-1wholeF1(ACAAATACCCCTGGCTCAGG)和MT-1wholesR1(ACCTGTTCCATAATCGTC),dmtA(登录号AB022906),以及用于钙调素基因的CMDF1(GGTGGGCCAGACACAC)和CMDR1(CCGATGGGGGGGTCATGACGTG)(登录号AY017584)。 喂养实验。[1] 通过尖端培养法,在28°C下,在添加或不添加2 mgcyclo(l-Leu-l-Pro)ml−1的情况下,将寄生A.NIAH-26在添加了40μM杂色曲霉素的YES培养基中培养4天。如上所述,通过用氯仿提取培养基,然后进行HPLC分析来测量黄曲霉毒素的形成。 |
| 细胞实验 |
细胞毒性试验(MTT试验)[3]
化合物对癌症细胞系(包括HT-29、MCF-7、A375、K562和NCM460细胞系)的细胞毒性活性使用具有小修饰的MTT测定法进行测量。简而言之,将细胞以每孔1×104个细胞的密度铺在96孔板上,并让其生长过夜,然后用含有连续稀释化合物的新鲜RPMI1640培养基替换培养基,并在37°C和5%CO2下进一步孵育48小时。在这个实验中,1和6被稀释成6个不同的系列浓度,从10μg/mL到0.0097μg/mL,每个稀释4倍,而2和3被稀释成500μg/mL到2.06μg/mL,4、5和7/Cyclo(Pro-Leu) 从300μg/mL到1.23μg/mL,用3×每次稀释。紫杉醇是一种用作阳性对照的临床药物,用两个系列稀释液从100μg/mL稀释至3.125μg/mL。HT-29、MCF-7和A375均在无血清RPMI1640中培养,而K562在整个实验过程中在含FBS的DMEM中培养。孵育后取出培养基,然后向每个孔中加入100μL 5mg/mL MTT溶液,在37°C下进一步孵育4小时。最后,取出上清液,向每个孔加入150μL二甲亚砜(DMSO)。在室温下摇动板10分钟后,使用微孔板读数器在570nm处用分光光度法测量光密度值。测定IC50值(50%抑制浓度)以评估这些细胞系的细胞存活率。在本实验中,使用Graph Pad Prism 5软件计算半抑制浓度(IC50)值。每个实验独立重复至少三次,表示为平均值±标准差(SD)。 抑制性物质 (e.g. cyclo(l-Leu-l-Pro))的活性测试。[1] (i) 目视琼脂平板试验。[1] 为了选择抑制黄曲霉毒素产生的细菌,对NA积累突变体NFRI-95进行了轻微修改,使用了目视琼脂平板试验(14)(图1A)。将真菌孢子接种在含有GY琼脂的平板中心的一条线上,然后将细菌液体培养物的等分试样接种在距离中心线1.5cm的线上。在28°C下孵育3至7天后,从平板下侧观察细菌对菌丝体中NA积累或真菌生长的影响。真菌菌丝体中红色素(NA)的减少表明细菌抑制了黄曲霉毒素的产生。 (ii)微量滴定琼脂平板试验。[1] 基于视觉琼脂平板试验,我们设计了一个小规模的试验系统,其中使用96孔平底组织培养板作为培养容器(图1B和C)。将高压灭菌的GY琼脂(100μl)倒入每个孔中,然后固化。将每个纯化步骤的每个组分的等分试样(10至20μl)或含有抑制物质的甲醇溶液施加到琼脂培养基上,然后在没有盖子的情况下孵育30分钟以上,使溶液扩散到培养基中,甲醇蒸发。将A.parasiticus NFRI-95的孢子悬浮液(1μl)接种到培养基的中心。因为孢子的数量不影响结果,所以我们没有针对这种方法进行调整。在板的每个角落放置一小块粘土或Parafilm,在盖子和板之间形成一个薄间隙,并用手术胶带(21N,12号;12毫米乘9米)密封该间隙,使盖子不会打开。在28°C下孵育2或3天后,从平板下侧观察菌丝体的颜色。 (iii)Tip culture method [1] 使用移液管作为培养容器,将寄生曲霉SYS-4的孢子悬浮液(5μl)接种到250μl补充了不同浓度抑制物质的YES培养基中。在28°C下孵育4天后,通过离心分离菌丝体和培养基。为了检测黄曲霉毒素,将5μl培养基点样到薄层色谱(TLC)硅胶板(硅胶60)上,然后用含有氯仿、乙酸乙酯和90%甲酸(6:3:1,体积/体积/体积)的溶液展开。黄曲霉毒素在长波长紫外光(365 nm)下进行检测,并在紫外光(365纳米)下用Fluor-S MultiImager拍照。为了测量黄曲霉毒素的量,用氯仿提取培养滤液,并将所得氯仿提取物的等分试样注入岛津高效液相色谱(HPLC)装置(型号LC-10A),该装置配备有硅胶柱(0.46×15cm;Shim-pack CLC-SI)和荧光监测器(激发波长,365nm;发射波长,425nm;岛津型号RF-535),流速为1ml min-1,在室温下。溶剂体系由甲苯、乙酸乙酯、甲酸和甲醇(178:15:4:3,体积/体积/体积)组成。将黄曲霉毒素B1、B2、G1和G2的保留时间与标准代谢物样品(黄曲霉毒素B-黄曲霉毒素G混合物)的保留时间进行了比较。为了检测黄曲霉毒素的前体,用丙酮提取菌丝垫中的前体。提取物浓缩至干后,将碎片溶解在苯乙腈(98:2,体积比)中,然后通过TLC进行分析。 |
| 动物实验 |
In vivo antimalarial screening of bioactive compound [2]
ICR strain male mice (n=120, 25–30 g, 6–8 weeks old) were used in all animal experiments and were divided into 20 groups (n=6). All groups were housed in stainless steel cages under 12:12 hours with and without light conditions at 28°C with daily ad libitum feed. To initiate the infection, 0.1 mL of 1.0×106 P. berghei PZZ1/100 parasitized red blood cell (RBC) solution was intraperitoneously administered into the host. To determine the best concentration, 50, 25, 12.5, 6.25 and 3.125 μg kg−1 bw of cyclo(l-Leu-l-Pro) or gancidin W/GW solutions were prepared by dissolving the obtained fractions of cyclo(l-Leu-l-Pro) or gancidin W/GW in 1.0 mL dimethyl sulfoxide (DMSO) as the stock compound solution before they were serially diluted with sterile distilled water to achieve the targeted concentrations. The 4-day suppression test was chosen to implement the antimalarial screening, whereby day zero of the treatment was the day when the mice were treated with 0.1 mL of cyclo(l-Leu-l-Pro) or gancidin W/GW at all concentrations, immediately within 2 hours postinfection. In parallel, 0.1 mL of 10 mg kg−1 bw of dH2O-diluted quinine hydrochloride (QH) and 0.9% normal saline were respectively used as positive control (PC) and negative control (NC) solutions. After 20 days postinfection, the mice were observed daily for their survival period (days). Treatment regime with inhibition rate of >65% was considered as having antimalarial activity, and the mice group with the longest survival time was considered as receiving the best treatment. In vivo toxicity assessment of the compound [2] Mice with the same characteristics as during antimalarial screening (ICR strain, male, 25–30 g, 6–8 weeks old, n=36) were used for in vivo toxicity assessment. All animal experiments for toxicity assessment were conducted under the same Universiti Kebangsaan Malaysia Animal Ethics Committee (UKMAEC) approval code for antimalarial screening. The mice were divided into 12 groups (n=6), and all groups were subjected to the same conditions (28°C room temperature, stainless steel cage, 12:12 hours with and without light and daily ad libitum feed). At the best-detected concentration during antimalarial screening, toxicity tests were carried out on blood samples of mice treated with cyclo(l-Leu-l-Pro) or gancidin W/GW according to two types of toxicity regime: acute exposure (daily treatment for 7 days) and subacute exposure (daily treatment for 28 days). Each toxicity regime was divided into two groups of treatment: without infection and immediately within 2 hours after infection on day zero. For labeling purposes, all the mice groups were respectively labeled as TA for acute exposure without infection, TB (acute exposure immediately within 2 hours after infection), TC (subacute exposure without infection) and TD (subacute exposure immediately within 2 hours after infection). Data from the two control regimens, namely, normal mice without any infection and treatment (CN) and single-dose infected mice (CI), were also obtained for comparison. The animals were sacrificed under diethyl ether anesthesia, 0.8–1.0 mL of the blood was collected from each mouse by cardiac puncture on day eight and day 29 postexposure and tested for serum total protein (STP), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) levels. From the same diethyl ether-anesthetized mice used for biochemical toxicity tests, three vital organs, namely, liver, kidney and spleen, were individually collected for organ toxicity and histology studies. The infiltration procedure was done automatically by a tissue processor. The tissues were next embedded in hot paraffin wax using an embedding machine. These tissues were then cut (0.4–0.6 μm thick) by a microtome. Histologic tissue preparation was done using hematoxylin-and-eosin (H–E) staining method. The H–E-stained slides for organ histology were observed using a computerized light microscopic camera at 100× magnification by placing a drop of immersion oil on the surface of the slide or without the immersion oil at 40× magnification; this method was vital to give a clear picture of the internal structure of the targeted tissues, as well as for assessing and identifying abnormalities, toxicity and injuries in the tissues. |
| 参考文献 |
|
| 其他信息 |
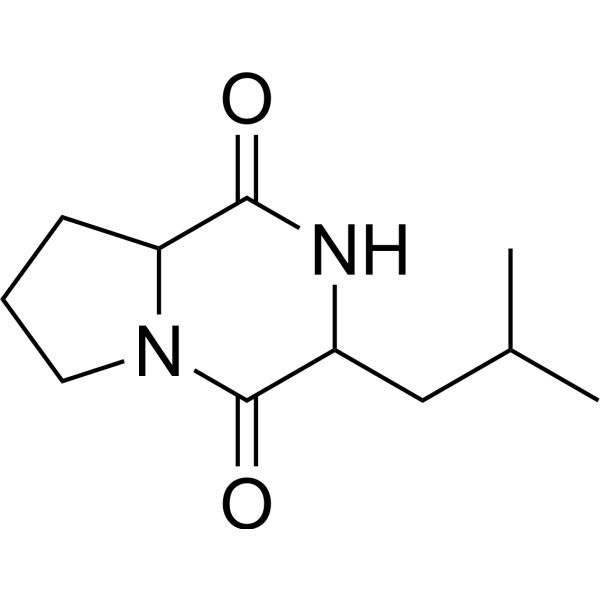
3-(2-methylpropyl)-octahydropyrrolo[1,2-a]pyrazine-1,4-dione is an organonitrogen compound and an organooxygen compound. It is functionally related to an alpha-amino acid.
3-Isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione has been reported in Streptomyces malaysiense, Streptomyces nigra, and other organisms with data available. Cyclo(L-Leu-L-Pro) is a homodetic cyclic peptide composed from leucyl and prolyl residues. It has a role as a marine metabolite and a bacterial metabolite. It is a dipeptide, a homodetic cyclic peptide and a pyrrolopyrazine. ChEBI (3S,8aS)-3-Isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione has been reported in Epichloe typhina, Peroneutypa scoparia, and other organisms with data available. A new aliphatic acid, compound 1, together with six known metabolites, including nonactic acid (2), homononactic acid (3), ethyl homononactate (4), homononactylhomononactate (5), valinomycin (6), and Cyclo(Pro-Leu) (7), was isolated from the culture broth of Streptomyces sp. BM-8, an actinobacterial strain isolated from the feces of Equus quagga. The structures of these compounds were established by analyses of spectroscopic data, including 1D and 2D nuclear magnetic resonance spectra (NMR), as well as by HR-ESI-MS spectrometry and chemical derivative analyses. Additionally, a serial analogue of nonactic acid and homononacticacid (8-21) was synthesized. The cytotoxicity of 1-21 wastested against a panel of cancer cell lines, such as HT-29, MCF-7, A375 and K562, with MTT assay. In addition, the cytotoxicity tests revealed that 1 was less cytotoxic toward a panel of cancerous cells, as compared with valinomycin (6).[3] Aflatoxins are potent carcinogenic and toxic substances that are produced primarily by Aspergillus flavus and Aspergillus parasiticus. We found that a bacterium remarkably inhibited production of norsolorinic acid, a precursor of aflatoxin, by A. parasiticus. This bacterium was identified as Achromobacter xylosoxidans based on its 16S ribosomal DNA sequence and was designated A. xylosoxidans NFRI-A1. A. xylosoxidans strains commonly showed similar inhibition. The inhibitory substance(s) was excreted into the medium and was stable after heat, acid, or alkaline treatment. Although the bacterium appeared to produce several inhibitory substances, we finally succeeded in purifying a major inhibitory substance from the culture medium using Diaion HP20 column chromatography, thin-layer chromatography, and high-performance liquid chromatography. The purified inhibitory substance was identified as cyclo(l-Leu-l-Pro) based on physicochemical methods. The 50% inhibitory concentration for aflatoxin production by A. parasiticus SYS-4 (= NRRL2999) was 0.20 mg ml(-1), as determined by the tip culture method. High concentrations (more than 6.0 mg ml(-1)) of cyclo(L-leucyl-L-prolyl) further inhibited fungal growth. Similar inhibitory activities were observed with cyclo(D-leucyl-D-prolyl) and cyclo(L-valyl-L-prolyl), whereas cyclo(D-prolyl-L-leucyl) and cyclo(L-prolyl-D-leucyl) showed weaker activities. Reverse transcription-PCR analyses showed that cyclo(l-Leu-l-Pro) repressed transcription of the aflatoxin-related genes aflR, hexB, pksL1, and dmtA. This is the first report of a cyclodipeptide that affects aflatoxin production. [1] In purifying the inhibitor, we found that the culture medium of the A1 bacterium contained at least three inhibitory substances other than cyclo(l-Leu-l-Pro). The amounts of these substances were not large enough for further characterization. Although the combination of these unknown substances and cyclo(l-Leu-l-Pro) might result in synergetic inhibitory activity, detailed relationships among the substances remain to be studied. The biosynthetic mechanism and the secretion mechanism of the cyclodipeptide of A. xylosoxidans NFRI-A1 are still unknown. It has been reported that cyclodipeptides might be degradation products of proteins following spontaneous cyclization. However, it has been found that many cyclodipeptides play various biological roles. These substances are now regarded as important metabolic substances rather than as protein artifacts. For purification of the inhibitor from the bacterial culture, a simple, sensitive, and small-scale detection system is of primary importance. Although the visual agar plate assay provided sensitive and clear results, the experimental scale seemed to be too large even if we used a small plate (diameter, 6 cm). Instead, we devised a small-scale assay system using a microtiter plate. The microtiter plate is quite useful. Magnusson et al. have independently reported use of a similar microtiter plate well assay to monitor antifungal activity of the metabolites produced by Lactobacillus coryniformis subsp. coryniformis. We have already isolated other inhibitory substances from soil bacteria by using the microtiter agar plate assay. We hope that some of our results will soon be useful in preventing aflatoxin contamination.[1] |
| 分子式 |
C11H18N2O2
|
|---|---|
| 分子量 |
210.272822856903
|
| 精确质量 |
210.136
|
| 元素分析 |
C, 62.83; H, 8.63; N, 13.32; O, 15.22
|
| CAS号 |
5654-86-4
|
| 相关CAS号 |
Cyclo(L-Leu-L-Pro);2873-36-1; 274680-11-4; 5654-86-4; 32510-93-3
|
| PubChem CID |
102892
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
1.1
|
| tPSA |
49.4
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
15
|
| 分子复杂度/Complexity |
288
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(C)CC1C(=O)N2CCCC2C(=O)N1
|
| InChi Key |
SZJNCZMRZAUNQT-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C11H18N2O2/c1-7(2)6-8-11(15)13-5-3-4-9(13)10(14)12-8/h7-9H,3-6H2,1-2H3,(H,12,14)
|
| 化学名 |
3-(2-methylpropyl)-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione
|
| 别名 |
5654-86-4; Cyclo(Pro-Leu); 3-Isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione; Cyclo(leucyloprolyl); 3-(2-methylpropyl)-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione; 3-isobutyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione; Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)-; Cyclo(leucylprolyl);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.7558 mL | 23.7790 mL | 47.5579 mL | |
| 5 mM | 0.9512 mL | 4.7558 mL | 9.5116 mL | |
| 10 mM | 0.4756 mL | 2.3779 mL | 4.7558 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。