| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Acetylcholinesterase
|
|---|---|
| 体外研究 (In Vitro) |
盐酸普托品(10-40 μM,24-96 小时)抑制肝癌细胞(HepG2、Huh7)的 EMT 过程、迁移、侵袭和活力 [2]。盐酸普托品(10-40 μM,24 h)抑制 PI3K/Akt 信号通路,增加 HepG2 和 Huh7 细胞中 caspase-3 和 caspase-9 的产生,从而诱导细胞凋亡 [2]。在 HepG2 和 Huh7 细胞中,盐酸丙哌平(10–40 μM,6 小时)会导致 ROS 生成 [2]。盐酸普托品 (0–10 μg/mL) 会降低 N1 细胞中去甲肾上腺素 (NE) 的吸收和 S6 细胞中血清素转运蛋白 (SERT) 的吸收 [3]。
|
| 体内研究 (In Vivo) |
腹腔注射0.1和1 mg/kg盐酸普托品可改善1 mg/kg东莨菪碱引起的小鼠记忆障碍[1]。盐酸普托品(5–20 mg/kg,腹膜内注射)可抑制异种移植 BALB/c 小鼠(皮下注射 Huh-7 或 HepG2 细胞)的肿瘤生长、PI3K/Akt 和 caspase-3 裂解[2]。在小鼠 HTR 和 TST 测试中,盐酸普托品(5-20 mg/kg,腹腔注射)表现出类似于抗抑郁药的作用[3]。局灶性脑缺血损伤大鼠腹腔注射盐酸普托品(1-4mg/kg,每天一次,连续3天)反应较好[4]。
|
| 酶活实验 |
Protopine是一种异喹啉生物碱,具有多种生物活性,包括抗肿瘤活性。然而,普罗托品对肝癌细胞的影响仍然难以捉摸。本研究旨在检测普罗托品对肝癌细胞的体外和体内作用。
方法:采用MTT法测定细胞活力。进行伤口愈合和transwell检测以评估细胞的运动能力。流式细胞术检测细胞凋亡和ROS水平。Western印迹法用于测量蛋白质的变化。还评估了普罗托品在异种移植物小鼠中的细胞毒性。
结果:Protopine以半胱天冬酶依赖的方式通过内在途径抑制肝癌细胞的存活并触发凋亡。此外,普罗托品还诱导细胞内ROS的积累,进一步导致PI3K/Akt信号通路的抑制。最后,体内研究表明,普罗托品也抑制了异种移植物小鼠的肿瘤生长,没有明显的毒性。
结论:Protopine可作为治疗肝癌的潜在药物[2]。
|
| 细胞实验 |
蛋白质印迹分析[2]
细胞类型: HepG2、Huh7 测试浓度: 10、20、40 μM 孵育时间: 24小时 实验结果:诱导caspase-3和caspase-9的裂解。 Bcl-2 和 Bcl-xl 水平降低。诱导线粒体蛋白细胞色素 c 释放到细胞质中。 |
| 动物实验 |
Animal/Disease Models: 5-Hydroxy-DL-tryptophan (5-HTP)-induced mouse model [3]
Doses: 5, 10, 20 mg/kg Route of Administration: intraperitoneal (ip) injection Experimental Results: Increased 5-HTP-induced head Number of hemispheric twitch responses (HTR). Reduce the immobility time tested in the Tail Suspension Test (TST). The protopine isolated from a Chinese herb Dactylicapnos scandens Hutch was identified as an inhibitor of both serotonin transporter and noradrenaline transporter in vitro assays. 5-hydroxy-DL-tryptophan(5-HTP)-induced head twitch response (HTR) and tail suspension test were adopted to study whether protopine has anti-depression effect in mice using reference antidepressant fluoxetine and desipramine as positive controls. In HTR test, protopine at doses of 5, 10, 20 mg/kg dose dependently increase the number of 5-HTP-induced HTR. Protopine at doses of 3.75 mg/kg, 7.5 mg/kg and 30 mg/kg also produces a dose-dependent reduction in immobility in the tail suspension test. The present results open up new possibilities for the use of protopine in the treatment of mood disorders, such as mild and moderate states of depression.[3] Protopine, an isoquinoline alkaloidis, is known to produce many effects such as vasodilation, down-regulation of glutamate levels in brain and decrease of intracellular calcium. However, so far there is no report on the effect of protopine in cerebral ischaemia. In this study, the effect of protopine on the focal cerebral ischaemia was investigated in rats. Male Sprague-Dawley rats were divided into five groups: sham-operated group, vehicle-treated group and three doses of protopine-treated groups (0.98, 1.96 and 3.92 mg/kg). Protopine was intraperitoneally administered to rats once daily for 3 days prior to the ischaemia and 0.9% normal saline to rats in the vehicle-treated group in the same pattern. Rats in the sham-operated group were given 0.9% normal saline without the ischaemia. The focal cerebral ischaemia was induced by the middle cerebral artery occlusion for 24 hr via the intraluminal filament technique. The results showed that pre-treatment with protopine reduced the cerebral infarction ratio and serum lactate dehydrogenase activity, and improved the ischaemia-induced neurological deficit score and histological changes of brain in a dose-dependent manner. The further studies demonstrated that protopine increased superoxide dismutase activity in serum, and decreased total calcium and terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL)-positive cells in the ischaemic brain tissue in the middle cerebral artery occlusion rats. The results indicate that protopine is able to produce an effective protection on the injury caused by the focal cerebral ischaemia in rats possibly through the multiple effects of calcium antagonism, antioxidation and depression of cell apoptosis.[4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Xiang-Fu-Si-Wu Decoction (XFSWD) has been widely used to treat primary dysmenorrhea in clinical practice for hundreds of years and shown great efficacy. One fraction of XFSWD, which was an elution product by macroporous adsorption resin from aqueous extract solution with 60% ethanol (XFSWE), showed great analgesic effect. The present study was conducted to investigate the possible pharmacokinetic and tissue distribution profiles of four major bioactive constituents (berberine, protopine, tetrahydrocoptisine and tetrahydropalmatine) after oral administration of XFSWE in dysmenorrheal symptom rats, and to compare the difference between normal and dysmenorrheal symptom rats. Estradiol benzoate and oxytocin were used to produce dysmenorrheal symptom rat model. The experimental period was seven days. At the final day of experimental period, both normal and dysmenorrheal symptom rats were orally administrated with XFSWE, and then the blood and tissues samples were collected at different time points. Berberine, protopine, tetrahydrocoptisine and tetrahydropalmatine in blood and tissue samples were determined by LC-MS/MS. Pharmacokinetic parameters were calculated from the plasma concentration-time data using non-compartmental methods. The differences of pharmacokinetic parameters among groups were tested by one-way analysis of variance (ANOVA). There were statistically significant differences (P<0.05) in Cmax, Tmax, AUC(0-t), AUC(0-infinity), MRT(0-t), MRT(0-infinity) and CL/F between normal and dysmenorrheal symptom rats that orally administered with same dosage of XFSWE. In tissue distribution study, the results showed that the overall trend was C(Spleen)>C(Liver)>C(Kidney)>C(Uterus)>C(Heart)>C(Lung)>C(Ovary)>C(Brain)>C(Thymus), C(M-60 min)>C(M-120 min)>C(M-30 min)>C(C-60 min)>C(C-120 min)>C(C-30 min). The contents of protopine in liver, spleen and uterus were more than that in other tissues of dysmenorrheal symptom rats. Compared to normal rats, partial contents of the compounds in dysmenorrheal symptom rats' tissues at different time points had significant difference (P<0.05). This study was the first report about pharmacokinetic and tissue distribution investigation in dysmenorrheal symptom animals. The results indicated that berberine, protopine, tetrahydrocoptisine and tetrahydropalmatine have higher uptake and slower elimination in the rats with dysmenorrheal syndrome, which suggests that the rate and extent of drug metabolism were altered in dysmenorrheal syndrome rats. And the results also demonstrated that berberine, protopine and tetrahydropalmatine in normal and dysmenorrheal symptom rats had obvious differences in some organs and time points, suggesting that the blood flow and perfusion rate of the organ were altered in dysmenorrheal symptom animals. PMID:24837303 Metabolism / Metabolites Eschscholtzia californica preparations are in use as phytopharmaceuticals and as herbal drugs. Studies are described on the metabolism and the toxicological analysis of the Eschscholtzia californica alkaloids californine and protopine in rat urine using gas chromatography-mass spectrometry. ... Protopine ... undergoes extensive demethylenation of the 2,3-methylenedioxy group followed by catechol-O-methylation. All phenolic hydroxy metabolites were found to be partly conjugated. The authors' systematic toxicological analysis procedure using full-scan gas chromatography-mass spectrometry after acid hydrolysis, liquid-liquid extraction and microwave-assisted acetylation allowed the detection of the main metabolites of californine and protopine in rat urine after a dose which should correspond to that of drug users. Therefore, use of Eschscholtzia californica preparations should also be detectable in human urine by the authors' systematic toxicological analysis procedure. Xiang-Fu-Si-Wu Decoction (XFSWD) has been widely used to treat primary dysmenorrhea in clinical practice for hundreds of years and shown great efficacy. One fraction of XFSWD, which was an elution product by macroporous adsorption resin from aqueous extract solution with 60% ethanol (XFSWE), showed great analgesic effect. The present study was conducted to investigate the possible pharmacokinetic and tissue distribution profiles of four major bioactive constituents (berberine, protopine, tetrahydrocoptisine and tetrahydropalmatine) after oral administration of XFSWE in dysmenorrheal symptom rats, and to compare the difference between normal and dysmenorrheal symptom rats. Estradiol benzoate and oxytocin were used to produce dysmenorrheal symptom rat model. The experimental period was seven days. At the final day of experimental period, both normal and dysmenorrheal symptom rats were orally administrated with XFSWE, and then the blood and tissues samples were collected at different time points. Berberine, protopine, tetrahydrocoptisine and tetrahydropalmatine in blood and tissue samples were determined by LC-MS/MS. Pharmacokinetic parameters were calculated from the plasma concentration-time data using non-compartmental methods. The differences of pharmacokinetic parameters among groups were tested by one-way analysis of variance (ANOVA). There were statistically significant differences (P<0.05) in Cmax, Tmax, AUC(0-t), AUC(0-infinity), MRT(0-t), MRT(0-infinity) and CL/F between normal and dysmenorrheal symptom rats that orally administered with same dosage of XFSWE. In tissue distribution study, the results showed that the overall trend was C(Spleen)>C(Liver)>C(Kidney)>C(Uterus)>C(Heart)>C(Lung)>C(Ovary)>C(Brain)>C(Thymus), C(M-60 min)>C(M-120 min)>C(M-30 min)>C(C-60 min)>C(C-120 min)>C(C-30 min). The contents of protopine in liver, spleen and uterus were more than that in other tissues of dysmenorrheal symptom rats. Compared to normal rats, partial contents of the compounds in dysmenorrheal symptom rats' tissues at different time points had significant difference (P<0.05). This study was the first report about pharmacokinetic and tissue distribution investigation in dysmenorrheal symptom animals. The results indicated that berberine, protopine, tetrahydrocoptisine and tetrahydropalmatine have higher uptake and slower elimination in the rats with dysmenorrheal syndrome, which suggests that the rate and extent of drug metabolism were altered in dysmenorrheal syndrome rats. And the results also demonstrated that berberine, protopine and tetrahydropalmatine in normal and dysmenorrheal symptom rats had obvious differences in some organs and time points, suggesting that the blood flow and perfusion rate of the organ were altered in dysmenorrheal symptom animals. Eschscholtzia californica preparations are in use as phytopharmaceuticals and as herbal drugs. Studies are described on the metabolism and the toxicological analysis of the Eschscholtzia californica alkaloids californine and protopine in rat urine using gas chromatography-mass spectrometry. ... Protopine ... undergoes extensive demethylenation of the 2,3-methylenedioxy group followed by catechol-O-methylation. All phenolic hydroxy metabolites were found to be partly conjugated. The authors' systematic toxicological analysis procedure using full-scan gas chromatography-mass spectrometry after acid hydrolysis, liquid-liquid extraction and microwave-assisted acetylation allowed the detection of the main metabolites of californine and protopine in rat urine after a dose which should correspond to that of drug users. Therefore, use of Eschscholtzia californica preparations should also be detectable in human urine by the authors' systematic toxicological analysis procedure. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Protopine is a solid. It is used as medication. HUMAN EXPOSURE AND TOXICITY: Using gene reporter assays performed in transiently transfected HepG2 cells, it was demonstrated that the induction of CYP1A1 expression by protopine was associated with mild or negligible activation of the aryl hydrocarbon receptor. CYP1A mRNA levels induced by protopine in both HepG2 cells and human hepatocytes did not result in elevated CYP1A protein or activity levels. ANIMAL STUDIES: Protopine showed an ability to enhance gamma-aminobutyric acid binding to rat brain synaptic membrane receptors in in vitro radiolabeling studies. Protopine has antiarrhythmic effects and may directly inhibit rapid electrical activity of cardiac cells. Protopine has been found to inhibit histamine H1 receptors and platelet aggregation, and acts as an analgesic. It is one of the compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. Protopine can selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Classical antihistaminics antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. Protopine can also function as platelet aggregation inhibitors which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. Protopine inhibits the contractility of isolated cardiac papillary muscles and the proliferation of vascular smooth muscle cells induced by endothelin. It also shortens action potential duration and prolongs the effective refractory period in guinea pig cardiac papillary muscles. The protective effect on rat heart from ischemia_reperfusion damage and the relaxation of rat thoracic aorta induced by protopine have been related to the inhibition of Ca2+ influx through both voltage- and receptor-operated Ca2+ channels. Protopine has been the focus of a large number of biological studies in which they both exhibited, for instance, anti-parasitic activity and only weak cytotoxicity in comparison with other types of isoquinoline alkaloids. Protopine was found to be cytoprotective against oxidative stress induced cell death in vitro. The alkaloid was shown to have anti-arrhythmic, anti-thrombotic, anti-inflammatory, and hepatoprotective effects in animal models. The biological activity of protopine may be associated with its ability to inhibit calcium, sodium, and potassium channels. (PMID:15588728; PMID:21419197; L2104) Interactions The antiarrhythmic effects of protopine on experimental arrhythmia were studied in various animals. Protopine elevated the dose of aconitine needed to induce VP, VT, and VF in rats and increased the dose of strophanthin (strophanthine K) that induced VP in guinea pigs. It also shortened the duration of central arrhythmia induced by aconitine and the duration of arrhythmia induced by benzene-epinephrine (adrenaline) in rats. It prevented rats and mice from developing arrhythmia induced by intravenous calcium chloride and inhalation of chloroform, respectively. In rabbits, the drug raised VFT. It was concluded that protopine has antiarrhythmic effects and may directly inhibit rapid electrical activity of cardiac cells. Lu Z et al; Chin Pharm J (Zhongguo Yaoxue Zazhi); 30: 81-84 (REF 9) (1995) Antidote and Emergency Treatment /SRP:/ Immediate first aid: Ensure that adequate decontamination has been carried out. If patient is not breathing, start artificial respiration, preferably with a demand valve resuscitator, bag-valve-mask device, or pocket mask, as trained. Perform CPR if necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention. Non-Human Toxicity Values LD50 Guinea pig ip 116 mg/kg LD50 Guinea pig oral 237 mg/kg LD50 Mouse ip 482 mg/kg |
| 参考文献 |
|
| 其他信息 |
Mechanism of Action
CACL2 (0.2 G/KG, IV) INDUCED FIBRILLATION OF THE RAT CARDIAC VENTRICLES FOR 2 SEC AND CAUSED DEATH OF THE ANIMALS. PROTOPINE-HCL (10 MG/KG) PROLONGED THE VENTRICULAR FIBRILLATION TO 186 SEC. IT ALSO RESTORED THE SINUS RHYTHM 3 MIN AFTER ITS ADMIN IN ALL TREATED ANIMALS. |
| 分子式 |
C20H20CLNO5
|
|---|---|
| 分子量 |
389.8295
|
| 精确质量 |
389.103
|
| CAS号 |
6164-47-2
|
| 相关CAS号 |
Protopine;130-86-9
|
| PubChem CID |
22543
|
| 外观&性状 |
PRISMS FROM ALC
|
| 沸点 |
547.5ºC at 760 mmHg
|
| 熔点 |
208ºC
|
| 闪点 |
284.9ºC
|
| LogP |
3.297
|
| tPSA |
57.23
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
542
|
| 定义原子立体中心数目 |
0
|
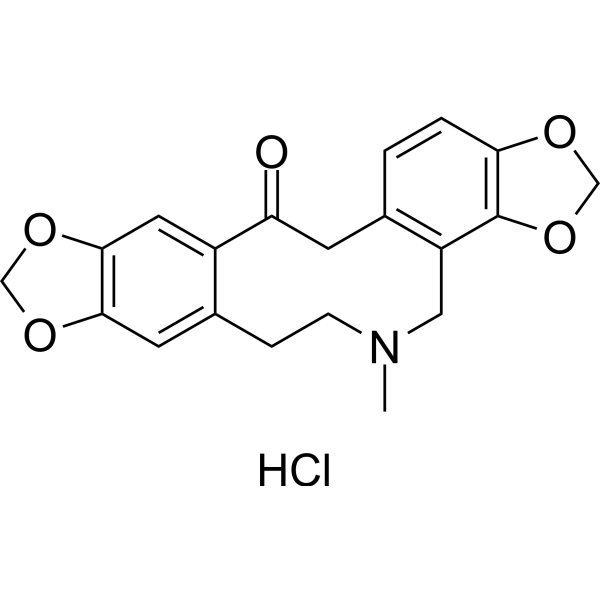
| SMILES |
Cl.CN1CCC2=CC3OCOC=3C=C2C(=O)CC2=C(C3OCOC=3C=C2)C1
|
| InChi Key |
NWNVDSJZGYDVQW-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H19NO5.ClH/c1-21-5-4-13-7-18-19(25-10-24-18)8-14(13)16(22)6-12-2-3-17-20(15(12)9-21)26-11-23-17;/h2-3,7-8H,4-6,9-11H2,1H3;1H
|
| 化学名 |
15-methyl-7,9,19,21-tetraoxa-15-azapentacyclo[15.7.0.04,12.06,10.018,22]tetracosa-1(17),4,6(10),11,18(22),23-hexaen-3-one;hydrochloride
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5652 mL | 12.8261 mL | 25.6522 mL | |
| 5 mM | 0.5130 mL | 2.5652 mL | 5.1304 mL | |
| 10 mM | 0.2565 mL | 1.2826 mL | 2.5652 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。