| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
Human Endogenous Metabolite
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Readily absorbed from the gastrointestinal tract, except in malabsorption syndromes. Vitamin B12 is absorbed in the lower half of the ileum. Each hydroxocobalamin molecule can bind one cyanide ion by substituting it for the hydroxo ligand linked to the trivalent cobalt ion, to form cyanocobalamin, which is then excreted in the urine. The possibility of direct transport of hydroxocobalamin from the nasal cavity into the cerebrospinal fluid after nasal administration in rats was investigated and the results were compared with a human study. Hydroxocobalamin was given to rats (n=8) both intranasally (214 ug/rat) and intravenously (49.5 ug/rat) into the jugular vein using a Vascular Access Port (VAP). Prior to and after drug administration, blood and cerebrospinal fluid samples were taken and analysed by radioimmunoassay. The AUCcerebrospinal fluid/AUCplasma ratio after nasal delivery does not differ from the ratio after intravenous infusion, indicating that hydroxocobalamin enters the cerebrospinal fluid via the blood circulation across the blood-brain barrier (BBB). This same transport route is confirmed by the cumulative AUC-time profiles in cerebrospinal fluid and plasma, demonstrating a 30 min delay between plasma absorption and cerebrospinal fluid uptake of hydroxocobalamin in rats and in a comparative human study. The present results in rats show that there is no additional uptake of hydroxocobalamin in the cerebrospinal fluid after nasal delivery compared to intravenous administration, which is in accordance with the results found in humans. Fifty percent of the administered dose of hydroxocobalamin disappears from the injection site in 2.5 hours. Hydroxocobalamin is bound to plasma proteins and stored in the liver. It is excreted in the bile and undergoes some enterohepatic recycling. Within 72 hours after injection of 500 to 1000 mcg of hydroxocobalamin, 16 to 66 percent of the injected dose may appear in the urine. The major portion is excreted within the first 24 hours. Hydroxocobalamin is absorbed more slowly from the site of injection than is cyanocobalamin and there is some evidence that liver uptake of hydroxocobalamin may be greater than that of cyanocobalamin. It is believed that the increased retention of hydroxocobalamin compared with that of cyanocobalamin results from the greater affinity of hydroxocobalamin for both specific and nonspecific binding proteins in blood and tissues, as well as to its slower absorption from the injection site. In the presence of gastric acid and pancreatic proteases, dietary vitamin B12 is released from food and salivary binding protein and bound to gastric intrinsic factor. When the vitamin B12-intrinsic factor complex reaches the ileum, it interacts with a receptor on the mucosal cell surface and is actively transported into circulation. Adequate intrinsic factor, bile, and sodium bicarbonate (to provide a suitable pH) all are required for ileal transport of vitamin B12. Vitamin B12 deficiency in adults is rarely the result of a deficient diet per se; rather, it usually reflects a defect in one or another aspect of this complex sequence of absorption. Achlorhydria and decreased secretion of intrinsic factor by parietal cells secondary to gastric atrophy or gastric surgery is a common cause of vitamin B12 deficiency in adults. Antibodies to parietal cells or intrinsic factor complex also can play a prominent role in producing a deficiency. A number of intestinal diseases can interfere with absorption, including pancreatic disorders (loss of pancreatic protease secretion), bacterial overgrowth, intestinal parasites, sprue, and localized damage to ileal mucosal cells by disease or as a result of surgery. /Vitamin B-12/ For more Absorption, Distribution and Excretion (Complete) data for HYDROXOCOBALAMIN (9 total), please visit the HSDB record page. Metabolism / Metabolites Primarily hepatic. Cobalamins are absorbed in the ileum and stored in the liver. They continuously undergo enterohepatic recycling via secretion in the bile. Part of a dose is excreted in the urine, most of it in the first 8 hours. Toxicokinetics of hydroxocobalamin were studied in rats and in dogs after single administration. In dogs, the AUCs of free cobalamins-(III) and total cobalamins-(III) increased proportionally to the dose. Mean Cmax measured for free- and total cobalamins-(III) were 1 to 5 fold higher than those measured in humans treated with 5.0 and 10.0 g hydroxocobalamin. Terminal half-lives reached approximately 6 and 8 hours for free and total cobalamins-(III), respectively in dogs. Corresponding figures in rats amounted to 3 and 5 hours. In dogs, the clearance of total cobalamins-(III) (0.064 to 0.083 L/h/kg) was 6-7 fold lower than clearance of free cobalamins-(III). The binding of hydroxocobalamin to proteins may be regarded as reversible metabolism. Hydroxocobalamin also reacts with cyanide thereby forming cyanocobalamin. This complex is highly stable and is therefore regarded as a physiological end product of hydroxocobalamin especially during cyanide intoxication. Primarily hepatic. Cobalamins are absorbed in the ileum and stored in the liver. They continuously undergo enterohepatic recycling via secretion in the bile. Part of a dose is excreted in the urine, most of it in the first 8 hours. Cobalt is absorbed though the lungs, gastrointestinal tract, and skin. Since it is a component of the vitamin B12 (cyanocobalamin), it is distributed to most tissues of the body. It is transported in the blood, often bound to albumin, with the highest levels being found in the liver and kidney. Cobalt is excreted mainly in the urine and faeces. (L29) Route of Elimination: Each hydroxocobalamin molecule can bind one cyanide ion by substituting it for the hydroxo ligand linked to the trivalent cobalt ion, to form cyanocobalamin, which is then excreted in the urine. Half Life: Approximately 6 days (peak plasma concentration after 8-12 hours from oral administration) Biological Half-Life Approximately 6 days (peak plasma concentration after 8-12 hours from oral administration) In normal individuals, hydroxocobalamin has a plasma half life of 3-20 hours. In patients with cyanide poisoning, the half life is 14-24 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Vitamin B12 exists in four major forms referred to collectively as cobalamins; deoxyadenosylcobalamin, methylcobalamin, hydroxocobalamin, and cyanocobalamin. Two of these, methylcobalamin and 5-deoxyadenosyl cobalamin, are primarily used by the body. Methionine synthase needs methylcobalamin as a cofactor. This enzyme is involved in the conversion of the amino acid homocysteine into methionine. Methionine in turn is required for DNA methylation. 5-Deoxyadenosyl cobalamin is a cofactor needed by the enzyme that converts L-methylmalonyl-CoA to succinyl-CoA. This conversion is an important step in the extraction of energy from proteins and fats. Furthermore, succinyl CoA is necessary for the production of hemoglobin, the substances that carries oxygen in red blood cells. Protein Binding Very high (90%). Cobalamins are extensively bound to two specific plasma proteins called transcobalamin 1 and 2; 70% to transcobalamin 1, 5% to transcobalamin 2. Toxicity Data LD50: >50mL/kg (i.v., mice) (L1865) Interactions Concurrent administration of chloramphenicol and vitamin B12 reportedly may antagonize the hematopoietic response to vitamin B12 in vitamin B12-deficient patients. The hematologic response to vitamin B12 in patients receiving both drugs should be carefully monitored and alternate anti-infectives should be considered. /Vitamin B12/ Prednisone has been reported to increase the absorption of vitamin B12 and secretion of intrinsic factor (IF) in a few patients with pernicious anemia, but not in patients with partial or total gastrectomy. The clinical importance of these findings is unknown. /Vitamin B12/ Ascorbic acid may destroy substantial amounts of dietary vitamin B12 in vitro; this possibility should be considered when large doses of ascorbic acid are ingested within 1 hour of oral vitamin B12 administration. /Vitamin B12/ Absorption of vitamin B12 from the GI tract may be decreased by aminoglycoside antibiotics, colchicine, extended-release potassium preparations, aminosalicylic acid and its salts, anticonvulsants (e.g., phenytoin, phenobarbital, primidone), cobalt irradiation of the small bowel, and by excessive alcohol intake lasting longer than 2 weeks. Neomycin-induced malabsorption of vitamin B12 may be increased by concurrent administration of colchicine. /Vitamin B12 Caution should be exercised when administering other cyanide antidotes simultaneously with Cyanokit, as the safety of coadministration has not been established. If a decision is made to administer another cyanide antidote with Cyanokit, these drugs should not be administered concurrently in the same IV line. Non-Human Toxicity Values LD50 Mouse iv 2 g/kg |
| 参考文献 |
|
| 其他信息 |
Hydroxocobalamin, also known as vitamin B12a and hydroxycobalamin, is an injectable form of vitamin B 12 that has been used therapeutically to treat vitamin B 12 deficiency. It is also used in cyanide poisoning, Leber's optic atrophy, and toxic amblyopia.
Hydroxocobalamin is an Antidote. Hydroxocobalamin is a synthetic form of vitamin B12 that can be used as a dietary supplement to treat vitamin B12 deficiency. Upon administration, hydroxocobalamin mimics vitamin B12 and acts as an essential cofactor in various cellular reactions required for cell growth and replication, and hematopoiesis. Hydroxocobalamin is only found in individuals that have used or taken this drug.It is an injectable form of vitamin B 12 that has been used therapeutically to treat vitamin B 12 deficiency. [PubChem] Injectable form of VITAMIN B 12 that has been used therapeutically to treat VITAMIN B 12 DEFICIENCY. See also: Hydroxocobalamin Acetate (active moiety of); Hydroxocobalamin Hydrochloride (is active moiety of). Drug Indication For treatment of pernicious anemia and the prevention and treatment of vitamin B12 deficiency arising from alcoholism, malabsorption, tapeworm infestation, celiac, hyperthyroidism, hepatic-biliary tract disease, persistent diarrhea, ileal resection, pancreatic cancer, renal disease, prolonged stress, vegan diets, macrobiotic diets or other restrictive diets. Also for the treatment of known or suspected cyanide poisoning. Treatment of known or suspected cyanide poisoning. Cyanokit is to be administered together with appropriate decontamination and supportive measures. Mechanism of Action Vitamin B12 exists in four major forms referred to collectively as cobalamins; deoxyadenosylcobalamin, methylcobalamin, hydroxocobalamin, and cyanocobalamin. Two of these, methylcobalamin and 5-deoxyadenosyl cobalamin, are primarily used by the body. Methionine synthase needs methylcobalamin as a cofactor. This enzyme is involved in the conversion of the amino acid homocysteine into methionine. Methionine in turn is required for DNA methylation. 5-Deoxyadenosyl cobalamin is a cofactor needed by the enzyme that converts L-methylmalonyl-CoA to succinyl-CoA. This conversion is an important step in the extraction of energy from proteins and fats. Furthermore, succinyl CoA is necessary for the production of hemoglobin, the substances that carries oxygen in red blood cells. Hydroxocobalamin is a complexation agent that acts by direct binding of the cyanide ions, resulting in cyanocobalamin which is a highly stable, nontoxic compound that is excreted in the urine. In addition, increased blood pressure observed in some healthy subjects of the phase I clinical study and results of a non-clinical study performed in anesthetized rabbits suggest an interference of hydroxocobalamin with the NO system. VITAMIN B12 IS IMPLICATED IN PROTEIN SYNTH THROUGH ITS ROLE IN SYNTH OF AMINE ACID METHIONINE... /COBALAMINS/ COENZYME B12 IS REQUIRED FOR HYDROGEN TRANSFER & ISOMERIZATION WHEREBY METHYLMALONATE IS CONVERTED TO SUCCINATE, THUS INVOLVING COBALAMIN IN BOTH FAT & CARBOHYDRATE METABOLISM. ... METHYLCOBALAMIN IS REQUIRED FOR CONVERSION OF HOMOCYSTEINE TO METHIONINE IN MAMMALS. /COBALAMINS/ Therapeutic Uses Hematinics Cyanokit is indicated for the treatment of known or suspected cyanide poisoning. Pernicious anemia, both uncomplicated and accompanied by nervous system involvement. The US government considers cyanide to be among the most likely agents of chemical terrorism. Cyanide differs from many other biological or chemical agents for which little or no defense is available because its individual and public health effects are largely remediable through appropriate preparedness and response. Because the toxicity of the cyanide antidote currently available in the United States renders it ill-suited for use in terrorist incidents and other situations requiring rapid out-of-hospital treatment, hydroxocobalamin--an effective and safe cyanide antidote being used in other countries--has been introduced in the United States. Unlike the other available cyanide antidote, hydroxocobalamin can be administered at the scene of a cyanide disaster, and it need not be reserved for cases of confirmed cyanide poisoning but can be administered in cases of suspected poisoning. Both of these attributes facilitate the rapid intervention necessary for saving lives. To realize the potential benefits of hydroxocobalamin, progress also needs to be realized in other aspects of readiness, including but not limited to developing plans for ensuring local and regional availability of antidote, educating emergency responders and health care professionals in the recognition and management of cyanide poisoning, and raising public awareness of the potential for a chemical weapons attack and of how to respond. For more Therapeutic Uses (Complete) data for HYDROXOCOBALAMIN (10 total), please visit the HSDB record page. Drug Warnings Caution should be exercised when administering other cyanide antidotes simultaneously with Cyanokit, as the safety of coadministration has not been established. If a decision is made to administer another cyanide antidote with Cyanokit, these drugs should not be administered concurrently in the same IV line. Use caution in the management of patients with known anaphylactic reactions to hydroxocobalamin or cyanocobalamin. Consideration should be given to use of alternative therapies, if available. Allergic reactions may include: anaphylaxis, chest tightness, edema, urticaria, pruritus, dyspnea, and rash. Allergic reactions including angioneurotic edema have also been reported in postmarketing experience. Maternal Medication usually Compatible with Breast-Feeding: B12: Reported Sign or Symptom in Infant or Effect on Lactation: None. /from Table 6/ While determination of blood cyanide concentration is not required for management of cyanide poisoning and should not delay treatment with Cyanokit, collecting a pretreatment blood sample may be useful for documenting cyanide poisoning as sampling post- Cyanokit use may be inaccurate. For more Drug Warnings (Complete) data for HYDROXOCOBALAMIN (19 total), please visit the HSDB record page. Pharmacodynamics Hydroxocobalamin is a synthetic, injectable form of Vitamin B12. Hydroxocobalamin is actually a precursor of two cofactors or vitamins (Vitamin B12 and Methylcobalamin) which are involved in various biological systems in man. Vitamin B12 is required for the conversion of methylmalonate to succinate. Deficiency of this enzyme could therefore interfere with the production of lipoprotein in myelin sheath tissue and so give rise to neurological lesions. The second cofactor, Methylcobalamin, is necessary for the conversion of homocysteine to methionine which is essential for the metabolism of folic acid. Deficiency of tetrahydrafolate leads to reduced synthesis of thymidylate resulting in reduced synthesis of DNA which is essential for cell maturation. Vitamin B12 is also concerned in the maintenance of sulphydryl groups in reduced form, deficiency leading to decreased amounts of reduced SH content of erythrocytes and liver cells. Overall, vitamin B12 acts as a coenzyme for various metabolic functions, including fat and carbohydrate metabolism and protein synthesis. It is necessary for growth, cell replication, hematopoiesis, and nucleoprotein as well as myelin synthesis. This is largely due to its effects on metabolism of methionine folic acid, and malonic acid. |
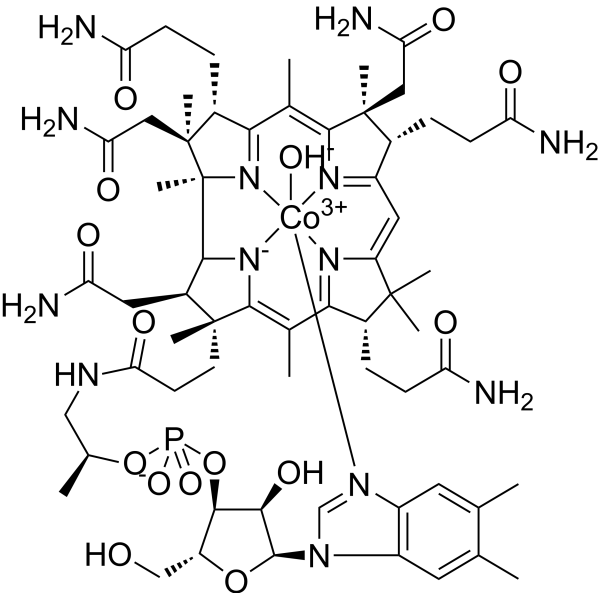
| 分子式 |
C62H89CON13O15P
|
|---|---|
| 分子量 |
1346.36
|
| 精确质量 |
1345.567
|
| CAS号 |
13422-51-0
|
| 相关CAS号 |
Hydroxocobalamin monohydrochloride;59461-30-2;Hydroxocobalamin acetate;22465-48-1;Hydroxocobalamin hydrochloride;58288-50-9
|
| PubChem CID |
44475014
|
| 外观&性状 |
Purple to black solid powder
|
| 熔点 |
200ºC (decomposes)
|
| LogP |
6.438
|
| tPSA |
484.1
|
| 氢键供体(HBD)数目 |
10
|
| 氢键受体(HBA)数目 |
20
|
| 可旋转键数目(RBC) |
26
|
| 重原子数目 |
92
|
| 分子复杂度/Complexity |
3140
|
| 定义原子立体中心数目 |
14
|
| SMILES |
CC1=CC2=C(C=C1C)N(C=N2)[C@@H]3[C@@H]([C@@H]([C@H](O3)CO)OP(=O)([O-])O[C@H](C)CNC(=O)CC[C@@]\4([C@H]([C@@H]5[C@]6([C@@]([C@@H](C(=N6)/C(=C\7/[C@@]([C@@H](C(=N7)/C=C\8/C([C@@H](C(=N8)/C(=C4\[N-]5)/C)CCC(=O)N)(C)C)CCC(=O)N)(C)CC(=O)N)/C)CCC(=O)N)(C)CC(=O)N)C)CC(=O)N)C)O.[OH-].[Co+3]
|
| InChi Key |
YOZNUFWCRFCGIH-WZHZPDAFSA-K
|
| InChi Code |
InChI=1S/C62H90N13O14P.Co.H2O/c1-29-20-39-40(21-30(29)2)75(28-70-39)57-52(84)53(41(27-76)87-57)89-90(85,86)88-31(3)26-69-49(83)18-19-59(8)37(22-46(66)80)56-62(11)61(10,25-48(68)82)36(14-17-45(65)79)51(74-62)33(5)55-60(9,24-47(67)81)34(12-15-43(63)77)38(71-55)23-42-58(6,7)35(13-16-44(64)78)50(72-42)32(4)54(59)73-56;;/h20-21,23,28,31,34-37,41,52-53,56-57,76,84H,12-19,22,24-27H2,1-11H3,(H15,63,64,65,66,67,68,69,71,72,73,74,77,78,79,80,81,82,83,85,86);;1H2/q;+3;/p-3/t31-,34-,35-,36-,37+,41-,52-,53-,56-,57+,59-,60+,61+,62+;;/m1../s1
|
| 化学名 |
cobalt(3+);[(2R,3S,4R,5S)-5-(5,6-dimethylbenzimidazol-1-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl] [(2R)-1-[3-[(1R,2R,3R,4Z,7S,9Z,12S,13S,14Z,17S,18S,19R)-2,13,18-tris(2-amino-2-oxoethyl)-7,12,17-tris(3-amino-3-oxopropyl)-3,5,8,8,13,15,18,19-octamethyl-2,7,12,17-tetrahydro-1H-corrin-21-id-3-yl]propanoylamino]propan-2-yl] phosphate;hydroxide
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 25 mg/mL (18.57 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.7427 mL | 3.7137 mL | 7.4274 mL | |
| 5 mM | 0.1485 mL | 0.7427 mL | 1.4855 mL | |
| 10 mM | 0.0743 mL | 0.3714 mL | 0.7427 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。