| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
β-lactam; bacterial cell wall synthesis
|
|---|---|
| 体外研究 (In Vitro) |
头孢洛林酯(TAK-599)是抗甲氧西林耐药金黄色葡萄球菌(MRSA)头孢菌素2a (T-91825)的新型n-膦前药,对青霉素结合蛋白(PBP) 2′(IC(50)具有高亲和力;0.90微克/毫升),并显示出有效的体外抗MRSA活性(MIC抗MRSA N133;1.56微克/毫升),与万古霉素(1.56微克/毫升)相当。[1]
|
| 体内研究 (In Vivo) |
Ceftaroline fosamil 内盐 (sc) 对 S 诱导的实验性全身感染具有抗菌特性。在小鼠中观察到金黄色葡萄球菌 N133 的 ED50 为 1.60–2.37 mg/kg[1]。在大鼠和猴子的血液中,头孢洛林酯内盐(10 mg/kg;皮下注射)迅速消失并容易转化为 T-91825[1]。
|
| 酶活实验 |
以枯草芽孢杆菌为试验菌,抗生素培养基2为扩散培养基,采用微生物法测定活性ceftaroline/头孢他林浓度 (低检出限,0.25 mg/升;日内及日内变动<10%)。[2]
|
| 动物实验 |
Using the neutropenic lung infection model, 17 clinical S. aureus isolates (2 MSSA, 15 MRSA) are investigated. For a duration of 24 hours, groups of six mice are treated with Ceftaroline fosamil starting three hours after inoculation. Doses of ceftaroline fosamil are injected subcutaneously in increments of 0.2 mL. Normal saline is given to control animals in the same amounts, ways, and intervals as the treatment plans[1].
For ceftaroline, blood samples were taken from six healthy rabbits after administration of a ceftaroline acetate bolus of 10 and 30 mg/kg of body weight in order to determine the spontaneous drug kinetics. The simulation was intended to provide apparent values of pharmacokinetic parameters close to those observed in healthy volunteers after a 1-h infusion of a 600-mg dose (ca. 10 mg/kg) of ceftaroline acetate: mean half-life (t1/2), 1.57 to 2.63 h; peak concentration (Cmax), 18.96 to 21.02 mg/liter; and area under the curve (AUC), 56.08 mg·h/liter. A total dose of 58 mg/kg needed to be infused into the rabbit over a 12-h period in order to simulate the kinetics in human serum after the administration of a 10-mg/kg dose (i.e., 600 mg twice daily). For each MRSA strain, the animals were randomly assigned to either no treatment (controls), ceftaroline regimen mimicking the human dose of 10 mg/kg every 12 h (q12h) (600 mg q12h), a linezolid regimen mimicking the human dose of 10 mg/kg q12h (600 mg q12h), and vancomycin administered by a constant intravenous infusion in order to reach a steady-state 20× MIC in serum. Experimental endocarditis was induced with an inoculum of 108 CFU of S. aureus. Treatment was started 24 h after inoculation for a 4-day regimen. Aortic valve vegetations were excised, weighed, and then homogenized in 0.5 ml of saline buffer and used for quantitative cultures on agar for 24 h at 37°C. Dilutions at 10−1, 10−2, and 10−4 were prepared to eliminate potential carryover effects. To evaluate whether ceftaroline treatment could induce the selection of variants resistant in vivo, undiluted vegetation homogenates were spread on agar plates containing the active form of ceftaroline at a concentration corresponding to fourfold the MIC. Bacterial counts were determined after 48 h of incubation at 37°C.[2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
primarily eliminated by the kidneys (6% in feces within 48 hours). Median 20.3 L (18.3-21.6 L). Metabolism / Metabolites Ceftaroline fosamil is converted into bioactive ceftaroline in plasma by a phosphatase enzyme. Hydrolysis of the beta-lactam ring of ceftaroline occurs to form the microbiologically inactive, open-ring metabolite ceftaroline M-1. Biological Half-Life 1.60 hours (600 mg dose). |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Although no information is available on the use of ceftaroline during breastfeeding, cephalosporins are generally not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Ceftaroline is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding approximately 20%. |
| 参考文献 |
|
| 其他信息 |
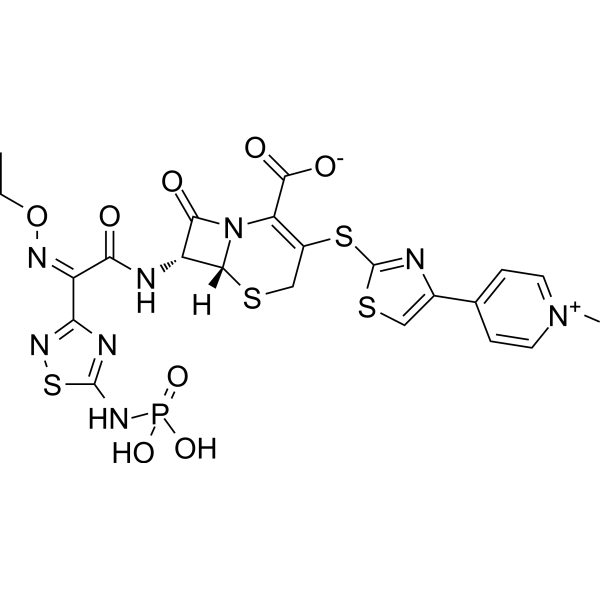
Ceftaroline fosamil is a cephalosporin having [4-(1-methylpyridinium-4-yl)-1,3-thiazol-2-yl]sulfanyl and {(2Z)-2-(ethoxyimino)-2-[5-(phosphonoamino)-1,2,4-thiadiazol-3-yl]acetyl}amino side groups located at positions 3 and 7 respectively. The N-phospho prodrug of ceftaroline, a broad-spectrum antibiotic active against methicillin-resistant Staphylococcus aureus (MRSA). It is used for the treatment of adults with acute bacterial skin and skin structure infections. It has a role as an antibacterial drug, an antimicrobial agent and a prodrug. It is an iminium betaine, a cephalosporin, a member of 1,3-thiazoles, an oxime O-ether, a member of thiadiazoles and an organic phosphoramidate. It is functionally related to a ceftaroline.
Ceftaroline fosamil is a cephalosporin antibacterial indicated for the treatment of the following infections caused by designated susceptible bacteria: Acute bacterial skin and skin structure infections. Community-acquired bacterial pneumonia. Ceftaroline Fosamil is an N-phosphono prodrug of the fifth-generation cephalosporin derivative ceftaroline with antibacterial activity. Ceftaroline fosamil is hydrolyzed to the active form ceftaroline in vivo. Ceftaroline binds to and inactivates penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall. PBPs are enzymes involved in the terminal stages of assembling the bacterial cell wall and in reshaping the cell wall during growth and division. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This results in the weakening of the bacterial cell wall and causes cell lysis. A fifth-generation cephalosporin antibacterial agent that is used to treat skin infections caused by bacteria in adults and newborns. Ceftaroline has a 1,3-thiazolidine ring attached to the C-7 position of its cephalosporin core. See also: Ceftaroline (has active moiety). Drug Indication Ceftaroline fosamil is indicated for the treatment of patients with the following infections caused by susceptible isolates of the designated microorganisms. FDA Label Zinforo is indicated for the treatment of the following infections in neonates, infants, children, adolescents and adults: , , , Complicated skin and soft tissue infections (cSSTI), Community-acquired pneumonia (CAP), , , Consideration should be given to official guidance on the appropriate use of antibacterial agents. , Treatment of community-acquired pneumonia, Treatment of complicated skin and soft-tissue infections Mechanism of Action Ceftaroline fosamil is an antibacterial drug. Pharmacodynamics The time that unbound plasma concentration of ceftaroline exceeds the minimum inhibitory concentration (MIC) of the infecting organism has been shown to best correlate with efficacy in a neutropenic murine thigh infection model with S. aureus and S. pneumoniae. No significant effect on QTc (corrected QT interval) interval was detected at peak plasma concentration or at any other time. |
| 分子式 |
C22H22N8O8PS4+
|
|---|---|
| 分子量 |
684.68
|
| 精确质量 |
685.018
|
| CAS号 |
229016-73-3
|
| 相关CAS号 |
Ceftaroline fosamil;400827-46-5; 229016-73-3; 400827-55-6
|
| PubChem CID |
9852981
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
1.419
|
| tPSA |
344.02
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
17
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
43
|
| 分子复杂度/Complexity |
1210
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CCO/N=C(\C(N[C@@H]1C(N2C(C(O)=O)=C(SC3=NC(C4=CC=[N+](C=C4)C)=CS3)CS[C@H]12)=O)=O)/C5=NC(SN5)=NP(O)([O-])=O
|
| InChi Key |
ZCCUWMICIWSJIX-NQJJCJBVSA-N
|
| InChi Code |
InChI=1S/C22H21N8O8PS4/c1-3-38-26-13(16-25-21(43-28-16)27-39(35,36)37)17(31)24-14-18(32)30-15(20(33)34)12(9-40-19(14)30)42-22-23-11(8-41-22)10-4-6-29(2)7-5-10/h4-8,14,19H,3,9H2,1-2H3,(H4-,24,25,27,28,31,33,34,35,36,37)/b26-13-/t14-,19-/m1/s1
|
| 化学名 |
(6R,7R)-7-[[(2Z)-2-ethoxyimino-2-[5-(phosphonoamino)-1,2,4-thiadiazol-3-yl]acetyl]amino]-3-[[4-(1-methylpyridin-1-ium-4-yl)-1,3-thiazol-2-yl]sulfanyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
|
| 别名 |
T-91825; T 91825; TAK599; TAK-599; 229016-73-3; Ceftaroline inner salt; CHEBI:70718; T91825; Teflaro; Zinforo;TAK 599;PP 0903; PPI-0903;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4605 mL | 7.3027 mL | 14.6054 mL | |
| 5 mM | 0.2921 mL | 1.4605 mL | 2.9211 mL | |
| 10 mM | 0.1461 mL | 0.7303 mL | 1.4605 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。