| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
MDM2
|
|---|---|
| 体外研究 (In Vitro) |
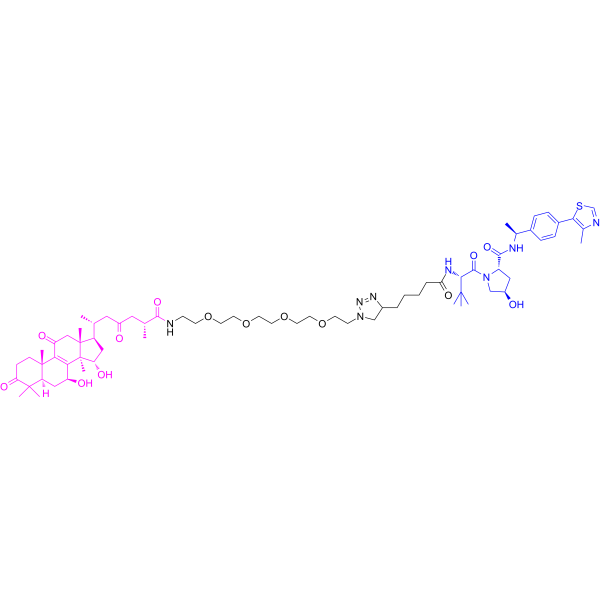
本研究设计合成了治疗乳腺癌的新型GAA PROTACs C1-C10和V1-V10两个系列。这些化合物对四种人肿瘤细胞系(MCF-7、MDA-MB-231、SJSA-1和HepG2)的抗肿瘤活性进行了评价。其中,V9和V10对乳腺癌细胞具有较强的抗增殖作用,其中抗肿瘤剂-150 (V10)对MDA-MB-231细胞(TNBC)的选择性最好,比先导化合物GAA高5倍。初步构效分析显示,v系列GAA PROTACs的效果优于c系列,并且引入20 - 40o PEG连接体可显著提高其抗肿瘤活性。分子对接、表面等离子体共振(SPR)、细胞热移实验(CETSA)和Western blot研究表明,V9和V10均可与MDM2结合,并通过泛素-蛋白酶体系统降解MDM2蛋白。分子动力学模拟(MD)表明V10是一种双功能分子,它可以一端结合von Hippel-Lindau (VHL),另一端靶向MDM2。此外,V10促进p53突变体MDA-MB-231细胞中p21的上调,并通过下调bcl-2/bax比值和cyclin B1的表达诱导细胞凋亡[1]。
抗肿瘤剂-150(V10)在TNBC斑马鱼模型中表现出良好的抗肿瘤活性,50 μg/mL时抑制率为27.2%[1]。抗肿瘤剂-150(V10,10μM)促进MDA-MB-231细胞凋亡[1]。抗肿瘤剂-150(V10)治疗显著改变了MDA-MB-231细胞的G1期和S期[1]。 |
| 体内研究 (In Vivo) |
体内实验表明,抗肿瘤剂-150 (V10)对异种移植TNBC斑马鱼模型也表现出良好的肿瘤抑制活性,50 μg/mL时抑制率为27.2%。综上所述,我们的研究结果表明,V10在体外和体内对p53突变型乳腺癌具有抗肿瘤作用,可能作为未来TNBC发展的新型先导化合物[1]。
|
| 酶活实验 |
SPR [1]
利用分子相互作用分析仪检测化合物V9和V10与MDM2蛋白的相互作用。MDM2蛋白(50 μg/mL)固定在PCH传感器芯片上,用EDC/NHS混合物预活化600 s,流速为10 μL/min。用含有5% DMSO和0.005% Tween的PBST缓冲液将V9或V10稀释至0、3.13、6.25、12.5、25、50、100 μM。结合时间为400 s,流速为15 μL/min。解离时间为60 s,通过计算机拟合和稳态分析得到亲和常数KD值。 |
| 细胞实验 |
细胞活力测定[1]
采用MTT细胞增殖测定试剂盒检测化合物对MCF-7、MDA-MB-231、SJSA-1、HepG2和HT-22细胞的抗增殖活性。96孔板中加入体积为100 μL的细胞悬液,每孔约5 × 103个细胞。文化板块在37°C pre-cultured孵化器5%二氧化碳的体积百分率24 h。第二天,最初的媒介是丢弃,和100μL中含有不同浓度的棉酚衍生品或5μM的积极药物盐酸hydroxydaunorubicin(阿霉素)添加到96孔板,在孵化器和孵化48 h或72 h。政府的相应时间后,10μL MTT的解决方案是添加到每个。培养板在培养箱中孵育4 h后,丢弃原有培养基,再加入150 μL DMSO。低速摇培养板10分钟后,用酶标仪测定每孔490 nm处吸光度,并以对照细胞的百分比表示每孔细胞活力。[1] |
| 动物实验 |
Determination of MTC in zebrafish and establishment of xenograft tumor model[1]
Wild type AB strain zebrafish embryos were fed in fish culture water at 28 °C (Water quality: 200 mg of instant sea salt per 1 L of reverse osmosis water, conductivity 450–550 μS/cm, pH 6.5–8.5, and hardness 50–100 mg/L CaCO3), the experimental animal use license number was SYXK (Zhe) 2022–0004. This research was approved by the international certification of Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Wild type AB strain zebrafish were randomly selected 3 dpf days after fertilization in 6-well plates, and 30 zebrafish were treated in each well. In addition to the normal control group, the other concentration groups were given V10 in water solution, and the normal control group was set up with a capacity of 3 mL per well. After treatment at 35 °C for 2 days, the MTC of V10 in normal zebrafish was determined.[1] MDA-MB-231 cells labeled with CM-DiI were transplanted into the yolk sac of 2 dpf wild type AB strain zebrafish by microinjection, and about 200 cells were transplanted into each tail to establish a zebrafish tumor transplantation model. The model zebrafish was placed at 35 °C and cultured to 3 dpf. At 3 dpf, zebrafish with good consistency of transplanted tumor cells were selected under the microscope and randomly assigned to 6-well plates with 30 cells per well. V10 was given in water solution, the cisplatin concentration in positive control was 15.0 μg/mL, and the model control group was set up with a capacity of 3 mL per well. After treatment at 35 °C for 2 days, 10 zebrafish were randomly selected from each experimental group and photographed under a fluorescence microscope. Data were collected using NIS-Elements D 3.20 advanced image processing software to analyze the fluorescence intensity of tumor cells, and the anti-tumor growth efficacy of V10 was evaluated based on the statistical analysis results of this index. The statistical results were expressed as mean ± SE. |
| 参考文献 | |
| 其他信息 |
In conclusion, two series of GAA PROTACs C1–C10 and V1–V10 were designed and synthesized as potent MDM2 protein degraders for the treatment of breast cancer. Screening results showed that V9 and V10 had stronger anti-proliferative effects on breast cancer cells, including an inhibition rate of 83.1% for V10 in TNBC (MDA-MB-231 cells), which was better than the lead compound GAA. Structure-activity relationships indicated that the introduction of 2O–4O PEG linkers could significantly improve the antitumor activity. Further investigation of the degradation properties revealed that both V9 and V10 could bind to MDM2 and degrade the protein through the ubiquitin-proteasome system. Among them, V10 can degrade MDM2 and increase the expression of p21 in TNBC. MD Simulations results proved the ternary complex of MDM2/V10/VHL is stable. In addition, V10 promoted the downregulation of bcl-2/bax and cyclin B1 expression, induced cell arrest in G1-S and G2-M phases, and ultimately led to cell apoptosis. In vivo experiments showed that V10 exhibited lower toxicity and significant anticancer activity with an inhibition rate of 27.2% at a dose concentration of 50 μg/mL. Therefore, the GAA PROTACs can not only avoid the drug resistance caused by MDM2-p53 negative feedback through MDM2 protein degradation, but also overcome the treatment difficulty caused by p53 mutation or inactivation in TNBC (
Fig. 11
). Based on the above results, our study demonstrated that the GAA MDM2 degrader agent V10 designed using the PROTAC strategy can enhance the anti-tumor activity of GAA, and it can be further developed and investigated as a MDM2 protein degrader for the treatment of TNBC.[1]
|
| 分子式 |
C70H106N8O14S
|
|---|---|
| 分子量 |
1315.70
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 别名 |
V10; V-10
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.7601 mL | 3.8003 mL | 7.6005 mL | |
| 5 mM | 0.1520 mL | 0.7601 mL | 1.5201 mL | |
| 10 mM | 0.0760 mL | 0.3800 mL | 0.7601 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。