| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
p2x1 Receptor (IC50 = 68 nM); P2X2 Receptor (IC50 = 214 nM)
|
|---|---|
| 体外研究 (In Vitro) |
NAADP已被证明在从植物到哺乳动物细胞的广泛系统中充当第二信使。尽管它一直被认为是一种典型的第二信使,但最近的研究表明,它在细胞外应用时也是活跃的。也有人认为,NAADP可能对P2受体有直接作用,这是基于药理学试剂PPADS对细胞外NAADP响应的Ca2+信号的作用。因此,我们研究了PPADS是否可以直接作用于特征明确的海胆卵匀浆系统中NAADP诱导的细胞内Ca2+释放系统。事实上,PPADS及其结构类似物PPNDS能够与[32P]NAADP竞争结合位点,结合曲线显示,这两种化合物在低微摩尔范围内都显示出亲和力。与NAADP相比,PPADS的结合是可逆的。在荧光Ca2+释放实验中,PPADS能够竞争性拮抗NAADP诱导的Ca2+释放,IC50为20微M,而不影响其他Ca2+释放通道。这是海胆NAADP受体可逆竞争性拮抗剂的首次报道。此外,PPADS可能是研究NAADP信号传导的宝贵工具,也是合成强效和特异性拮抗剂的先导化合物[1]。
|
| 体内研究 (In Vivo) |
外周损伤引起的神经性疼痛与局部炎症以及局部募集的巨噬细胞、雪旺氏细胞和神经胶质细胞中一氧化氮合酶(NOS)和炎性细胞因子的过表达有关。我们在坐骨神经慢性压迫损伤诱导的单神经病小鼠模型中研究了一氧化氮合酶(NOS)和细胞因子在中枢(脊髓和丘脑)和周围神经系统(神经和背根神经节)中的时间过程和定位。ATP被认为是内源性疼痛介质。因此,我们还评估了嘌呤能信号传导在疼痛超敏反应中的作用,使用P2受体拮抗剂磷酸吡哆醛-6-偶氮苯基-2',4'-二磺酸(PPADS)对疼痛行为、NOS和细胞因子进行了评估。从损伤后第3天开始每天服用PPADS,剂量和时间依赖性地降低了触觉异常性疼痛和热痛觉过敏。PPADS(25mg/kg)完全逆转了伤害性超敏反应,同时降低了参与疼痛信号传导的外周(受伤的坐骨神经和L4-L6同侧背根神经节)和神经系统中枢台阶(L4-L6脊髓和丘脑)中NO/NOS系统和IL-1β的增加。IL-6仅在外周神经系统中过表达,PPADS延长给药可降低其在坐骨神经中的表达。总之,我们假设NO/NOS和IL-1β在这种神经病变模型中具有促痛感作用,嘌呤能拮抗剂通过抑制其过度活动来减轻疼痛超敏反应[3]。
|
| 酶活实验 |
电生理学[2]
在电压钳条件下,使用双电极放大器记录了cRNA注射卵母细胞的核苷酸诱发膜电流。当填充KCl(3M)时,细胞内微电极的电阻为1-2MΩ。用含有(mM)NaCl 110、KCl 2.5、HEPES 5、BaCl2 1.8、pH 7.4-7.5的细胞外溶液持续灌注卵母细胞(5 ml min-1)。所有记录均在室温(18°C)下在-60至-90 mV的保持电位下进行。电生理数据最初以3 kHz的频率进行滤波,以20 Hz的频率在连接到MP100WSW接口的计算机上捕获,并使用商业软件显示。 酶联免疫吸附试验(ELISA)评价脊髓背侧IL-1β含量[3] 通过使用酶联免疫吸附试验对假手术、CCI和PPADS治疗的CCI动物的脊髓进行IL-1β蛋白的定量测定。如上所述,采集L4-L6脊髓切片,快速冷冻并储存在-80°C下。将样品在0.25ml含有蛋白酶抑制剂混合物的冰冷磷酸盐缓冲盐水中均质化并离心。上清液用于测量IL-1β水平。采用Lowry法测定颗粒中的总蛋白含量。 对于IL-1β的测量,使用小鼠IL-1β的CytoSet Elisa试剂盒。捕获抗体和二次生物素化抗体的浓度分别为1.25和0.125μg/ml。重组蛋白产生的标准曲线范围为15-1000pg/ml。 链霉抗生物素蛋白过氧化物酶和四甲基联苯胺用于显色。用2N H2SO4停止显色反应,并在450nm处读取光密度。 |
| 细胞实验 |
卵母细胞制备和P2X受体表达[2]
用Tricaine(0.2%,wt/vol)麻醉非洲爪蟾,并通过断头处死(根据机构规定)。卵巢的解剖和切除,以及去卵爪蟾卵母细胞的制备,在其他地方已有详细描述[King等人,1997]。去卵泡卵母细胞不具有天然的P1或P2受体,否则可能会使激动剂活性的分析复杂化[King等人,1996a,b]。此外,去卵泡卵母细胞在很大程度上缺乏胞外ATP酶活性,从而避免了P2受体拮抗剂抑制胞外酶的复杂问题[Ziganshin等人,1995]。用编码大鼠P2X1或大鼠P2X3受体亚基的封端核糖核酸(cRNA,1mg/ml)在细胞质中注射(40nl)成熟卵母细胞(V期和VI期)。将注射的卵母细胞在18°C下在含有(mM)NaCl 110、KCl 1、NaHCO3 2.4、Tris-HCl 7.5、Ca(NO3)2 0.33、CaCl2 0.41、MgSO4 0.82的沐浴液(pH 7.5)中孵育48小时,并补充50μg/l的硫酸庆大霉素,以使受体完全表达,然后在4°C下储存长达12天。 |
| 动物实验 |
Drug treatment [3]
Pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid tetrasodium salt (PPADS) was dissolved in saline and used at doses of 6.25, 12.5 and 25mg/kg (0.1ml/10g). Doses were chosen according to those employed by Gourine et al. (2005) in order to attenuate fever and cytokine responses induced by lipopolysaccharide in rats. PPADS or saline was administered i.p. to neuropathic and sham-operated mice once a day for 11 days, starting from the third day after surgery. The effect of the acute administration of PPADS at the highest dose (25mg/kg) has been evaluated at both third and 14th day after lesion: behavioural evaluations were performed both 1 and 24h after administration. The same experimental protocol was applied in mice treated for 10 days with saline, i.e. 14 days after sciatic nerve ligation. Thermal hyperalgesia and mechanical allodynia [3] Responses to thermal and mechanical stimuli were measured before and 3, 7 and 14 days (24h after the last administration with PPADS or saline) after the surgical procedure. Measurements were performed on both the ipsilateral and contralateral hind paws of all mice by researchers who were blind to treatments. Thermal hyperalgesia was tested according to the Hargreaves procedure (Hargreaves et al., 1988), slightly modified by us for mouse, using a Plantar test apparatus. Briefly, mice were placed in smaller clear plexiglass cubicles and allowed to acclimatize. A constant intensity radiant heat source (beam diameter 0.5cm and intensity 20 I.R.) was aimed at the midplantar area of the hind paw. The time, in seconds (s), from initial heat source activation until paw withdrawal was recorded. Mechanical allodynia was assessed using the Dynamic Plantar Aesthesiometer. Animals were placed in a test cage with a wire mesh floor, and the rigid tip of a von Frey filament (punctate stimulus) was applied to the skin of the midplantar area of the hind paw. The filament exerted an increasing force, ranging up to 5g in 20s, starting below the threshold of detection and increasing until the animal removed its paw. Withdrawal threshold was expressed in grams. Withdrawal threshold of ipsilateral and contralateral paws was measured four times and the value was the mean of the four evaluations. Biochemical evaluations [3] The biochemical evaluations were performed on animals receiving the highest dose of PPADS (25mg/kg) always by researchers who were blind to treatments. At 3, 7 and 14 days following surgery, 24h after the last dose of saline or PPADS, nociceptive and mechanical thresholds were recorded. Immediately after behavioural evaluations, mice were anaesthetized with sodium pentobarbital (60mg/kg, i.p., 0.1ml/10g) and under dissecting microscope the ipsilateral sciatic nerve, proximal to the trifurcation (about 1cm), before the three ligatures in the CCI animals, the ipsilateral L4, L5 and L6 DRG, the lumbar dorsal spinal cord at L4–L6 level, and ipsilateral and contralateral thalamus were removed and immediately frozen in liquid nitrogen and stored at −80°C until the NOSs content and cytokine expression assay. In some experiments a small portion of ipsilateral sciatic nerve, proximal to the trifurcation, before three ligatures in the CCI animals, and lumbar spinal dorsal at L4–L6 level was used to prepare nuclear extracts, which were stored at −80°C until the transcription factor NF-κB was assayed. In other experiments, a small portion of sciatic nerve, between the ligatures in CCI animals and trifurcation, was stored at −80°C until the assay of myelin proteins. In view of the technical difficulty of measurement of NO, which requires accuracy in the time of sampling and prompt measurement immediately after sampling, due to the instability of NO and nitrite, we evaluated the level of NOSs (inducible and neuronal) to represent NO changes, as previously reported by Salake et al. (2000) and Wang et al. (2004). |
| 参考文献 |
|
| 其他信息 |
5'-phosphopyridoxal-6-azobenzene-2,4-disulfonic acid is an arenesulfonic acid that is pyridoxal 5'-phosphate carrying an additional 2,4-disulfophenylazo substituent at position 6. It has a role as a purinergic receptor P2X antagonist. It is an arenesulfonic acid, a member of azobenzenes, a member of methylpyridines, a monohydroxypyridine, a pyridinecarbaldehyde and an organic phosphate. It is functionally related to a pyridoxal 5'-phosphate. It is a conjugate acid of a 5'-phosphonatopyridoxal-6-azobenzene-2,4-disulfonate.
Platelet Aggregation Inhibitors: Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. Seven PPADS (Pyridoxal-5'-Phosphate 6-Azophenyl 2',4'-DiSulfonate) analogs were investigated at Group 1 P2X receptors expressed in Xenopus oocytes. All seven analogs potently inhibited P2X1 (IC50 range, 5-32 nM) and P2X3 (IC50 range, 22-345 nM), the two Group I P2X receptor subtypes. Analogs showed greater inhibitory activity where the pyridoxal moiety of PPADS contained a 5'-phosphonate group, rather than a 5'-phosphate group. Analogs also showed greater potency where disulfonate groups were removed from, or substituted at, the azophenyl moiety. The most active analog was MRS 2257 (pyridoxal-5'-phosphonate 6-azophenyl 3',5'-bismethylenephosphonate) at P2X1 (IC50, 5 nM) and P2X3 (IC50, 22 nM) receptors, being 14-fold and 10-fold more potent than PPADS itself. MRS 2257 produced a nonsurmountable inhibition when tested against a range of ATP concentrations, although blockade was reversed by about 85% after 20 minutes of washout. TNP-ATP and Ip5I were equipotent with MRS 2257 at P2X1 receptors, whereas TNP-ATP was 64-fold more potent than MRS 2257 at P2X3 receptors. In conclusion, the PPADS template can be altered at the pyridoxal and phenyl moieties to produce P2X1 and P2X3 receptor antagonists showing higher potency and greater degree of reversibility than the parent compound at these Group I P2X receptors.[2] |
| 分子式 |
C14H14N3O12PS2
|
|---|---|
| 分子量 |
511.38
|
| 精确质量 |
598.903
|
| 元素分析 |
C, 28.06; H, 1.68; N, 7.01; Na, 15.34; O, 32.04; P, 5.17; S, 10.70
|
| CAS号 |
149017-66-3
|
| 相关CAS号 |
207572-67-6; 192575-19-2; 149017-66-3
|
| PubChem CID |
4881
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.94g/cm3
|
| 折射率 |
1.729
|
| LogP |
3.779
|
| tPSA |
288.3
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
15
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
956
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
PNFZSRRRZNXSMF-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C14H14N3O12PS2/c1-7-13(19)9(5-18)10(6-29-30(20,21)22)14(15-7)17-16-11-3-2-8(31(23,24)25)4-12(11)32(26,27)28/h2-5,19H,6H2,1H3,(H2,20,21,22)(H,23,24,25)(H,26,27,28)
|
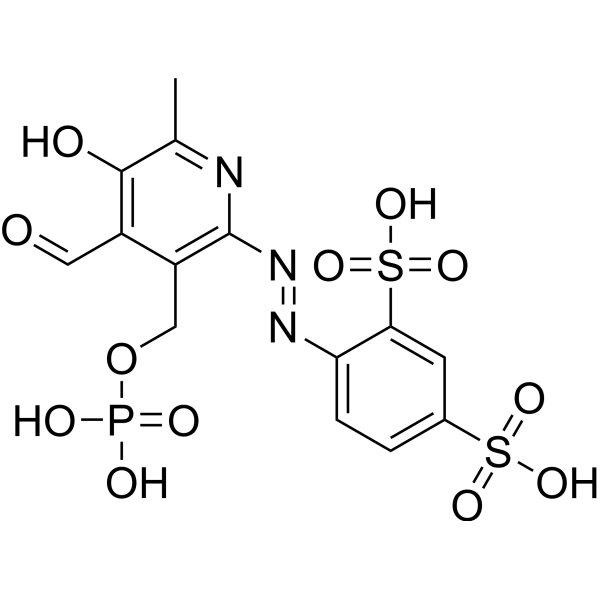
| 化学名 |
4-[[4-formyl-5-hydroxy-6-methyl-3-(phosphonooxymethyl)pyridin-2-yl]diazenyl]benzene-1,3-disulfonic acid
|
| 别名 |
ppads; 149017-66-3; Pyridoxal phosphate-6-azophenyl-2',4'-disulfonic acid; CHEBI:34941; L6K2LJ9BJK; CHEMBL69234; 4-((4-Formyl-5-hydroxy-6-methyl-3-((phosphonooxy)methyl)-2-pyridinyl)azo)-1,3-benzenedisulfonic acid; 4-[[4-formyl-5-hydroxy-6-methyl-3-(phosphonooxymethyl)pyridin-2-yl]diazenyl]benzene-1,3-disulfonic acid;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9555 mL | 9.7775 mL | 19.5549 mL | |
| 5 mM | 0.3911 mL | 1.9555 mL | 3.9110 mL | |
| 10 mM | 0.1955 mL | 0.9777 mL | 1.9555 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。