| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
5-HT2A Receptor ( Ki = 4 nM ); 5-HT1 Receptor ( Ki = 7 nM ); 5-HT6 Receptor ( Ki = 5 nM ); 5-HT2C Receptor ( Ki = 11 nM ); 5-HT3 Receptor ( Ki = 57 nM ); Adrenergic α1 Receptor ( Ki = 19 nM ); Muscarinic M1-5 Receptor ( Ki = 1.9-25 nM ); Dopamine Receptor; Mitophagy; Apoptosis
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:奥氮平与精神分裂症中感兴趣的关键受体相互作用,对多巴胺能、血清素能、α1-肾上腺素能和毒蕈碱受体具有纳摩尔亲和力。奥氮平的受体谱与氯氮平相似:它对多巴胺受体亚型相对无选择性,并且对中脑边缘和中皮质层比纹状体多巴胺束具有选择性(电生理学;Fos)。
奥氮平对其他受体、酶或神经元功能中的关键蛋白质几乎没有影响。奥氮平的受体谱与氯氮平相似:它对多巴胺受体亚型相对无选择性,对中脑边缘和中脑皮质的选择性高于纹状体多巴胺束(电生理学;Fos)。 结论:奥氮平的结合和功能特征(1)与氯氮平相似,(2)表明奥氮平是一种非典型抗精神病药物,(3)与临床疗效一致。如果奥氮平也被证明是安全的,那么它将很有可能成为一种更理想的抗精神病药物[1]。 |
||
| 体内研究 (In Vivo) |
奥氮平是 DA 受体(DOPAC 水平;培高利特刺激的血浆皮质酮增加)和 5-HT 受体(奎帕嗪刺激的皮质酮增加)的有效拮抗剂,但对 α-肾上腺素能和毒蕈碱受体的作用较弱。奥氮平与氟西汀联合使用可使大鼠前额皮质细胞外多巴胺 ([DA](ex)) 和去甲肾上腺素 ([NE](ex)) 水平强劲、持续增加,分别高达基线的 361% 和 272%,显着大于单独使用任何一种药物。 0.5 mg/kg、3 mg/kg 和 10 mg/kg (sc) 的奥氮平剂量依赖性地增加大鼠前额皮质、伏隔核和纹状体中的细胞外多巴胺 (DA) 和去甲肾上腺素 (NE) 水平。奥氮平还可增加 DA 代谢物 DOPAC 的细胞外水平以及释放的 DA 代谢物 3-甲氧基酪胺的组织浓度。奥氮平可使猕猴的平均新鲜脑重量以及左脑新鲜重量和体积减少 8-11%。奥氮平导致肥胖显着增加:全身脂肪增加反映了皮下和内脏脂肪储存的显着增加。奥氮平会导致明显的肝脏胰岛素抵抗。
体内奥氮平是DA受体(DOPAC水平;培高利特刺激的血浆皮质酮增加)和5-HT受体(喹嗪刺激的皮质酮减少)的强效拮抗剂,但在α肾上腺素能和毒蕈碱受体上较弱。[1] 为了了解Olanzapine/奥氮平和氟西汀联合治疗难治性抑郁症(TRD)的临床疗效机制,我们使用微透析研究了奥氮平及其他抗精神病药物联合选择性血清素摄取抑制剂氟西汀或舍曲林对大鼠前额叶皮层(PFC)神经递质释放的影响。奥氮平和氟西汀的组合使细胞外多巴胺([DA](ex))和去甲肾上腺素([NE](ex。这种组合产生的血清素([5-H](ex))增加量略小于单独使用氟西汀。氯氮平或利培酮与氟西汀联合使用时,[DA](ex)和[NE](ex。氟哌啶醇或MDL 100907与氟西汀的联合使用不会比单独使用氟西汀增加单胺。奥氮平联合舍曲林仅增加[DA](ex)。因此,奥氮平-氟西汀治疗后PFC中[DA](ex)、[NE](ex”)和[5-H](ex“)的大幅持续增加是独特的,可能有助于奥氮平和氟西汀治疗TRD的深刻抗抑郁作用。[3] 目前尚不清楚抗精神病药物治疗在多大程度上混淆了精神分裂症患者的纵向影像学研究和尸检。为了研究这个问题,我们开发了一种非人类灵长类动物慢性抗精神病药物暴露模型。三组每组六只猕猴分别口服氟哌啶醇、Olanzapine/奥氮平或假手术17-27个月。由此产生的血浆药物水平与用这些药物治疗的精神分裂症患者的水平相当。暴露后,我们观察到与假动物相比,两个药物治疗组的平均新鲜脑重量以及左侧大脑新鲜重量和体积减少了8-11%。在所有主要大脑区域(额叶、顶叶、颞叶、枕叶和小脑)都观察到了差异,但在额叶和顶叶区域表现得最为明显。使用Cavalieri原理对顶叶区域的体视学分析显示,灰质和白质的体积减少相似。此外,我们评估了标准组织学处理导致的后续组织收缩,没有发现药物暴露导致的差异性收缩的证据。然而,我们观察到大约20%的明显总体收缩效应,以及大脑各区域收缩的高度显著差异。总之,非人类灵长类动物长期接触抗精神病药物与脑容量减少有关。抗精神病药物可能会混淆依赖体积测量的精神分裂症患者的尸检和纵向影像学研究。[4] 非典型抗精神病药物与体重增加、高血糖和糖尿病有关。我们研究了非典型抗精神病药物奥氮平/Olanzapine(OLZ)和利培酮(RIS)与安慰剂对肥胖、胰岛素敏感性(S(I))和胰腺β细胞补偿的影响。狗被随意喂食,并服用OLZ(15mg/天;n=10)、RIS(5mg/天;n=10)或明胶胶囊(n=6)4-6周。OLZ导致肥胖显著增加:全身脂肪增加(+91+/-20%;P=0.000001),反映了皮下(+106+/-24%;P=0.0001)和内脏(+84+/-22%;P=0.00001)脂肪储存的显著增加。RIS组的肥胖变化与安慰剂组没有差异(P>0.33)。只有OLZ导致明显的肝胰岛素抵抗(肝S(I)[用药前后]:6.05+/-0.98 vs.1.53+/-0.93 dl。最小值(-1)。kg(-1)/[microU/ml];P=0.009)。β细胞敏感性在OLZ期间未能上调(用药前:1.24+/-0.15,用药后:1.07+/-0.25微U/ml(-1)/[mg/dl];P=0.6)。当将β细胞补偿与一组仅由中等脂肪喂养诱导的肥胖和胰岛素抵抗的动物进行比较时,进一步证明了OLZ诱导的β细胞功能障碍(+8%的热量来自脂肪;n=6)。这些结果可能解释非典型抗精神病药物的致糖尿病作用,并表明β细胞补偿受神经控制[5]。 |
||
| 酶活实验 |
方法:使用体外放射性受体结合的标准测定和成熟的体内(功能)测定来评估奥氮平与神经元受体的相互作用。
结果:结合研究表明,奥氮平与精神分裂症中感兴趣的关键受体相互作用,对多巴胺能、5-羟色胺能、α1-肾上腺素能和毒蕈碱受体具有纳摩尔亲和力[1]。 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Olanzapine presents a linear pharmacokinetic profile and, after daily administration, it reaches steady-state in about a week. Under the administration of a normal dosage of olanzapine, the steady-state plasma concentration does not seem to exceed 150 ng/ml with an AUC of 333 ng/h/ml. The absorption of olanzapine is not affected by the concomitant administration of food. The pharmacokinetic profile of olanzapine is characterized by reaching peak plasma concentration of 156.9 ng/ml approximately 6 hours after oral administration. Olanzapine is mainly eliminated through metabolism and hence, only 7% of the eliminated drug can be found as the unchanged form. It is mainly excreted in the urine which represents around 53% of the excreted dose followed by the feces that represent about 30%. The volume of distribution of olanzapine is reported to be of 1000 liters which indicate a large distribution throughout the body. The mean clearance rate of olanzapine is of 29.4 L/hour however, some studies have reported an apparent clearance of 25 L/h. The excretion of olanzapine into the breast milk of five lactating women with postpartum psychosis was examined in this study. Nine pairs of plasma and breast-milk samples were collected and the concentration of olanzapine determined by high-performance liquid chromatography. Single-point milk-to-plasma ratios were calculated and ranged from 0.2 to 0.84 with a mean of 0.46. The median relative infant dose was 1.6% (range 0-2.5%) of the weight-adjusted maternal dose. During the study period, there were no apparent ill effects on the infant as a consequence of exposure to these doses of olanzapine. As with other antipsychotic drugs this study demonstrates that olanzapine passes into breast milk. ... Olanzapine is distributed into milk. The manufacturer states that in a study in lactating, healthy women, the average infant dose of olanzapine at steady-state was estimated to be approximately 1.8% of the maternal olanzapine dose. In a separate study that evaluated the extent of infant exposure to olanzapine in 7 breastfeeding women who had been receiving 5-20 mg of olanzapine daily for periods ranging from 19-395 days, median and maximum relative infant doses of 1 and 1.2%, respectively, were observed. Olanzapine was not detected in the plasma of the breast-fed infants, and adverse effects possibly related to olanzapine exposure were not reported in the infants in this study. In addition, peak milk concentrations were achieved a median of 5.2 hours later than the corresponding maximal maternal plasma concentrations. In a case report, a relative infant dose of approximately 4% was estimated in one woman after 4 and 10 days (estimated to be at steady state) of olanzapine therapy at a dosage of 20 mg daily based on measurements of drug concentration in serum and in expressed breast milk. Intramuscular olanzapine for injection results in rapid absorption with peak plasma concentrations occurring within 15 to 45 minutes. Based upon a pharmacokinetic study in healthy volunteers, a 5 mg dose of intramuscular olanzapine for injection produces, on average, a maximum plasma concentration approximately 5 times higher than the maximum plasma concentration produced by a 5 mg dose of oral olanzapine. Area under the curve achieved after an intramuscular dose is similar to that achieved after oral administration of the same dose. The half-life observed after intramuscular administration is similar to that observed after oral dosing. The pharmacokinetics are linear over the clinical dosing range. Olanzapine is extensively distributed throughout the body, with a volume of distribution of approximately 1000 L. It is 93% bound to plasma proteins over the concentration range of 7 to 1100 ng/mL, binding primarily to albumin and alpha1-acid glycoprotein. Olanzapine is well absorbed and reaches peak concentrations in approximately 6 hours following an oral dose. It is eliminated extensively by first pass metabolism, with approximately 40% of the dose metabolized before reaching the systemic circulation. Food does not affect the rate or extent of olanzapine absorption. Pharmacokinetic studies showed that olanzapine tablets and olanzapine orally disintegrating tablets dosage forms of olanzapine are bioequivalent. Metabolism / Metabolites Olanzapine is greatly metabolized in the liver, which represents around 40% of the administered dose, mainly by the activity of glucuronide enzymes and by the cytochrome P450 system. From the CYP system, the main metabolic enzymes are CYP1A2 and CYP2D6. As part of the phase I metabolism, the major circulating metabolites of olanzapine, accounting for approximate 50-60% of this phase, are the 10-N-glucuronide and the 4'-N-desmethyl olanzapine which are clinically inactive and formed by the activity of CYP1A2. On the other hand, CYP2D6 catalyzes the formation of 2-OH olanzapine and the flavin-containing monooxygenase (FMO3) is responsible for N-oxide olanzapine. On the phase II metabolism of olanzapine, UGT1A4 is the key player by generating direct conjugation forms of olanzapine. Metabolic profiles after intramuscular administration are qualitatively similar to metabolic profiles after oral administration. Direct glucuronidation and cytochrome P450 (CYP) mediated oxidation are the primary metabolic pathways for olanzapine. In vitro studies suggest that CYPs 1A2 and 2D6, and the flavin-containing monooxygenase system are involved in olanzapine oxidation. CYP2D6 mediated oxidation appears to be a minor metabolic pathway in vivo, because the clearance of olanzapine is not reduced in subjects who are deficient in this enzyme. Following a single oral dose of (14)C labeled olanzapine, 7% of the dose of olanzapine was recovered in the urine as unchanged drug, indicating that olanzapine is highly metabolized. Approximately 57% and 30% of the dose was recovered in the urine and feces, respectively. In the plasma, olanzapine accounted for only 12% of the AUC for total radioactivity, indicating significant exposure to metabolites. After multiple dosing, the major circulating metabolites were the 10-N-glucuronide, present at steady state at 44% of the concentration of olanzapine, and 4'-N-desmethyl olanzapine, present at steady state at 31% of the concentration of olanzapine. Both metabolites lack pharmacological activity at the concentrations observed. Olanzapine has known human metabolites that include Olanzapine N-Oxide, 2-Hydroxymethyl Olanzapine, N-Desmethylolanzapine, and 7-Hydroxyolanzapine. Biological Half-Life Olanzapine presents a half-life ranging between 21 to 54 hours with an average half-life of 30 hours. Half-life ranges from 21 to 54 hours (5th to 95th percentile; mean of 30 hr) |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Olanzapine has a low potential for toxicity when prescribed alone. However, there are case reports which found olanzapine toxicity caused by high doses of the medication when taken in conjunction with other medicines. For example, a case report of a patient who overdosed by taking 560 milligrams of olanzapine in addition to 6.4 grams of propranolol and 280 milligrams of amlodipine had extreme hypotension, circulatory collapse, respiratory depression, and coma. According to the product labeling and postmarketing reports, the following are the features of olanzapine toxicity. Serum concentration of olanzapine >0.1 mg/L is toxic and serum concentration>1 mg/L can be fatal. Clinical Features * Agitation * Dysarthria * Tachycardia and hypotension * Extrapyramidal symptoms * Sedation * Miosis * Aspiration * Delirium * Respiratory depression * Coma * Convulsions * Ventricular dysrhythmia Management * There is no specific antidote to olanzapine. In acute overdosage, establish and maintain an airway and ensure adequate oxygenation and ventilation, including intubation. In addition, clinicians should consider the possibility of multiple drug involvement. * In addition, gastric lavage (after intubation, if the patient is unconscious) and administration of activated charcoal with a laxative should be considered. The administration of activated charcoal (1 g) reduced the Cmax and AUC of oral olanzapine by about 60%. As peak olanzapine levels are not typically obtained until about 6 hours after dosing, charcoal may be a valuable treatment for olanzapine overdose. * The possibility of obtundation, seizures, or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis. Therefore, cardiovascular monitoring should commence immediately and include continuous electrocardiographic monitoring to detect possible arrhythmias. * Hypotension and circulatory collapse should be treated with appropriate measures; intravenous fluids and sympathomimetic agents. Do not use epinephrine, dopamine, or other sympathomimetics with beta-agonist activity, since beta stimulation may worsen hypotension in the setting of the olanzapine-induced alpha blockade. * Close medical supervision and monitoring should continue until the patient recovers. Olanzapine's antipsychotic activity is likely due to a combination of antagonism at D2 receptors in the mesolimbic pathway and 5HT2A receptors in the frontal cortex. Antagonism at D2 receptors relieves positive symptoms while antagonism at 5HT2A receptors relieves negative symptoms of schizophrenia. Olanzapine is an antagonist at types 1, 2, and 4 dopamine receptors, 5-HT receptor types 2A and 2C, muscarinic receptors 1 through 5, alpha(1)-receptors, and histamine H1-receptors. Olanzapine's antipsychotic effect is due to antagonism at dopamine and serotonin type 2 receptors, with greater activity at serotonin 5-HT2 receptors than at dopamine type-2 receptors. This may explain the lack of extrapyramidal effects. Olanzapine does not appear to block dopamine within the tubero-infundibular tract, explaining the lower incidence of hyperprolactinemia than with typical antipsychotic agents or risperidone. Antagonism at muscarinic receptors, H1-receptors, and alpha(1)-receptors also occurs with olanzapine. Hepatotoxicity Liver test abnormalities have been reported to occur in 10% to 50% of patients on long term therapy with olanzapine. These abnormalities are usually mild, asymptomatic and transient, and can reverse even with continuation of medication. In addition, instances of more marked elevations in serum aminotransferase levels and clinically apparent hepatitis with jaundice have been reported in patients taking olanzapine. Among atypical antipsychotic agents, olanzapine has most often been linked to cases of clinically apparent liver injury, the incidence being estimated to be 1:1200 treated patients. The time to onset of liver injury with olanzapine therapy in generally within 1 to 4 weeks of starting therapy or achieving optimal daily dose. However, cases with onset a year after starting have also been reported. The pattern of serum enzyme elevations is most often mixed (Case) but can range from hepatocellular to cholestatic. Fatal cases of olanzapine induced liver injury have been reported, but most cases resolve rapidly once olanzapine is stopped. Allergic manifestations (rash, fever, eosinophilia) and autoimmune markers are uncommon. Cases with a long latency and accompanied by significant weight gain may represent nonalcoholic fatty liver disease, rather than olanzapine hepatotoxicity. Interactions The manufacturer states that the clearance of olanzapine in smokers is approximately 40% higher than in nonsmokers. Therefore, plasma olanzapine concentrations generally are lower in smokers than in nonsmokers receiving the drug. Adverse extrapyramidal effects have been reported in one olanzapine-treated patient after a reduction in cigarette smoking, while worsened delusions, hostility, and aggressive behavior have been reported in another olanzapine-treated patient following a marked increase in smoking (i.e., an increase from 12 up to 80 cigarettes per day). Although the precise mechanism(s) for this interaction has not been clearly established, it has been suggested that induction of the CYP isoenzymes, particularly 1A2, by smoke constituents may be responsible at least in part for the reduced plasma olanzapine concentrations observed in smokers compared with nonsmokers. Although the manufacturer states that routine dosage adjustment is not recommended in patients who smoke while receiving olanzapine, some clinicians recommend that patients treated with olanzapine should be monitored with regard to their smoking consumption and that dosage adjustment be considered in patients who have reduced or increased their smoking and/or who are not responding adequately or who are experiencing dose-related adverse reactions to the drug. In addition, monitoring of plasma olanzapine concentrations may be helpful in patients who smoke and have other factors associated with substantial alterations in metabolism of olanzapine (e.g., geriatric patients, women, concurrent fluvoxamine administration). Concurrent administration of activated charcoal (1 g) reduced peak plasma concentrations and the AUC of a single, 7.5-mg dose of olanzapine by approximately 60%. Since peak plasma concentrations are not usually obtained until about 6 hours after oral administration, activated charcoal may be useful in the management of olanzapine intoxication. Olanzapine therapy potentially may enhance the effects of certain hypotensive agents during concurrent use. In addition, the administration of dopamine, epinephrine, and/or other sympathomimetic agents with beta-agonist activity should be avoided in the treatment of olanzapine-induced hypotension, since such stimulation may worsen hypotension in the presence of olanzapine-induced alpha-blockade. In a pharmacokinetic study, concomitant administration of a single dose of alcohol did not substantially alter the steady-state pharmacokinetics of olanzapine (given in dosages of up to 10 mg daily). However, concomitant use of olanzapine with alcohol potentiated the orthostatic hypotension associated with olanzapine. The manufacturer therefore states that alcohol should be avoided during olanzapine therapy. For more Interactions (Complete) data for Olanzapine (11 total), please visit the HSDB record page. |
||
| 参考文献 |
[1]. J Clin Psychiatry. 1997:58 Suppl 10:28-36. [2]. Neuropsychopharmacology. 2000 Sep;23(3):250-62 [3]. Psychopharmacology (Berl). 1998 Mar;136(2):153-61. [4]. Neuropsychopharmacology. 2005 Sep;30(9):1649-61. [5]. Diabetes. 2005 Mar;54(3):862-71. [6]. APPROVED AGREED-UPON LABELING. [7]. Olanzapine for Injection, powder, for solution for intramuscular use. |
||
| 其他信息 |
Therapeutic Uses
Antiemetic, Antipsychotic Agent, Serotonin Uptake Inhibitor Oral olanzapine is indicated for the treatment of schizophrenia. Efficacy was established in three clinical trials in adult patients with schizophrenia: two 6-week trials and one maintenance trial. In adolescent patients with schizophrenia (ages 13-17), efficacy was established in one 6-week trial. /Included in US product label/ Oral olanzapine and fluoxetine in combination is indicated for the treatment of depressive episodes associated with bipolar I disorder, based on clinical studies in adult patients. /Included in US product label/ Oral olanzapine is indicated for the treatment of manic or mixed episodes associated with bipolar I disorder as an adjunct to lithium or valproate. Efficacy was established in two 6-week clinical trials in adults. The effectiveness of adjunctive therapy for longer-term use has not been systematically evaluated in controlled trials. /Included in US product label/ For more Therapeutic Uses (Complete) data for Olanzapine (7 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS. Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Olanzapine is not approved for the treatment of patients with dementia-related psychosis. A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with administration of antipsychotic drugs, including olanzapine. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac dysrhythmia). Additional signs may include elevated creatinine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure. The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to exclude cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system pathology. The management of NMS should include: 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy; 2) intensive symptomatic treatment and medical monitoring; and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for NMS. If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported. The possibility of a suicide attempt is inherent in schizophrenia and in bipolar I disorder, and close supervision of high-risk patients should accompany drug therapy. Prescriptions for olanzapine should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose. Like other atypical antipsychotic agents, olanzapine has a low potential for causing certain adverse extrapyramidal effects (e.g., dystonias). Results from controlled clinical trials suggest that extrapyramidal reactions associated with olanzapine therapy are dose related. Tremor was reported in about 4% of patients receiving oral olanzapine and in about 1% of patients receiving IM olanzapine in controlled clinical trials; the incidence of tremor appears to be dose related. In addition, akathisia occurred in about 3% of patients receiving oral olanzapine and in less than 1% of patients receiving IM olanzapine; hypertonia occurred in about 3% of patients receiving oral olanzapine in short-term controlled clinical trials. For more Drug Warnings (Complete) data for Olanzapine (45 total), please visit the HSDB record page. Pharmacodynamics The effect of olanzapine in the D2 receptor is reported to produce the positive effects of this drug such as a decrease in hallucinations, delusions, disorganized speech, disorganized thought, and disorganized behavior. On the other hand, its effect on the serotonin 5HT2A receptor prevents the onset of anhedonia, flat affect, alogia, avolition and poor attention. Based on the specific mechanism of action, olanzapine presents a higher affinity for the dopamine D2 receptor when compared to the rest of the dopamine receptor isotypes. This characteristic significantly reduces the presence of side effects. Clinical trials for the original use of olanzapine demonstrated significant effectiveness in the treatment of schizophrenia and bipolar disorder in adults and acute manic or mixed episodes associated with bipolar disorder in adolescents. The effect of olanzapine on dopamine and serotonin receptors has been suggested to reduce chemotherapy-induced nausea and vomiting as those receptors are suggested to be involved in this process. For this effect, several clinical trials have been conducted and it has been shown that olanzapine can produce a significant increase in total control of nausea and vomiting. In a high-level study of the effect of olanzapine for this condition, a complete response on the delay phase was observed in 84% of the individual and control of emesis of over 80% despite the phase. Background: Classical (typical) antipsychotic drugs are in wide use clinically, but some patients do not respond at all to treatment, while in others, negative symptoms and cognitive deficits fail to respond. Also, these drugs often cause serious motor disturbances. Clozapine, an atypical antipsychotic, appears to correct many of these deficiencies, but has a significant incidence of potentially fatal agranulocytosis. Accordingly, we attempted to develop a prototype of a new generation of antipsychotics that is both more efficacious and safe. Our strategy was to create a compound that is not only active in behavioral tests that predict antipsychotic action but also shares the rich, multifaceted receptor pharmacology of clozapine without its side effects. To this end, Eli Lilly and Co. developed olanzapine. In this article we characterize the in vitro and in vivo receptor pharmacology of olanzapine. Method: We evaluated olanzapine interactions with neuronal receptors using standard assays of radioreceptor binding in vitro and well-established in vivo (functional) assays. Results: Binding studies showed that olanzapine interacts with key receptors of interest in schizophrenia, having a nanomolar affinity for dopaminergic, serotonergic, alpha 1-adrenergic, and muscarinic receptors. In vivo olanzapine is a potent antagonist at DA receptors (DOPAC levels; pergolide-stimulated increases in plasma corticosterone) and 5-HT receptors (quipazine-stimulated increases in corticosterone), but is weaker at alpha-adrenergic and muscarinic receptors. Olanzapine has little or no effect at other receptors, enzymes, or key proteins in neuronal function. Olanzapine has a receptor profile that is similar to that of clozapine: it is relatively nonselective at dopamine receptor subtypes and it shows selectivity for mesolimbic and mesocortical over striatal dopamine tracts (electrophysiology; Fos). Conclusion: The binding and functional profile of olanzapine (1) is similar to that of clozapine, (2) indicates that olanzapine is an atypical antipsychotic drug, and (3) is consistent with clinical efficacy. If olanzapine also proves to be safe, then it will have high potential to become a more ideal antipsychotic drug.[1] In conclusion, this study is the first demonstration of the intrinsic effects of the most widely prescribed atypical antipsychotics on weight, adiposity, insulin sensitivity of the liver and peripheral tissues, and pancreatic β-cell function. There were clear differences in the effects of OLZ and RIS. OLZ caused significant weight gain and marked increases in total trunk adiposity, reflecting marked expansion of both visceral and subcutaneous adipose depots and severe hepatic insulin resistance. RIS had modest effects on adiposity that did not differ from the effects of placebo. Most importantly, the present studies reveal a significant effect of OLZ to impair β-cell compensation for insulin resistance. OLZ completely blocked the compensatory response with obesity and resistance seen with fat feeding, whereas β-cell function during RIS appears intact. The mechanisms by which these actions of antipsychotics occur are not known, but these data suggest that drugs may impede possible neural regulation of β-cell compensation. Failure of β-cell compensation to atypical antipsychotics provides a mechanistic basis by which diabetes may develop in the vulnerable psychiatric population treated with these therapeutic agents. These results underscore the importance of examining drug effects in the absence of risk factors common among psychiatric patients. Further studies are needed to determine the mechanisms underlying differential metabolic sequelae of these agents, and the processes which may lead to development of diabetes in this population.[5] |
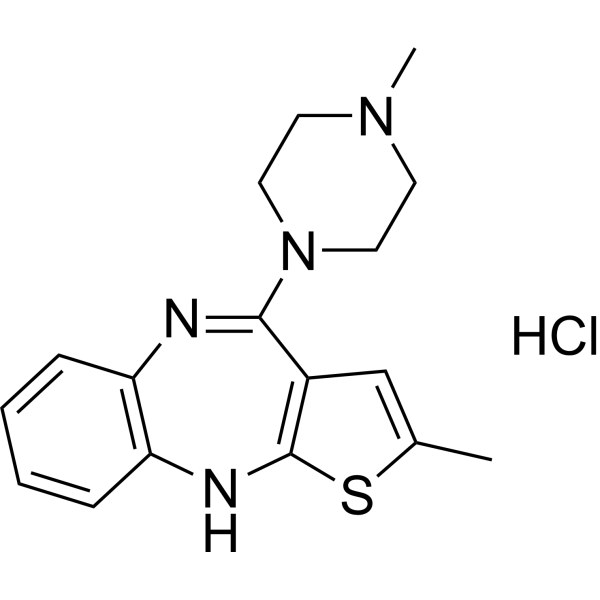
| 分子式 |
C17H21CLN4S
|
|---|---|
| 分子量 |
348.89
|
| 元素分析 |
C, 58.52; H, 6.07; Cl, 10.16; N, 16.06; S, 9.19
|
| CAS号 |
783334-36-1
|
| 外观&性状 |
Typically exists as solids at room temperature
|
| SMILES |
Cl.S1C(C)=CC2=C1NC1C=CC=CC=1N=C2N1CCN(C)CC1
|
| 别名 |
LY170053 hydrochloride; Olanzapine hydrochloride; UNII-0Q8K2L0MC3; 0Q8K2L0MC3; 783334-36-1; 10H-Thieno(2,3-b)(1,5)benzodiazepine, 2-methyl-4-(4-methyl-1-piperazinyl)-, hydrochloride (1:1); OLANZAPINE HYDROCHLORIDE [WHO-DD]; Olanzapine HCl; SCHEMBL1375329;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8662 mL | 14.3312 mL | 28.6623 mL | |
| 5 mM | 0.5732 mL | 2.8662 mL | 5.7325 mL | |
| 10 mM | 0.2866 mL | 1.4331 mL | 2.8662 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
The Impact of Preoperative Olanzapine on Quality of Recovery After Discharge from Ambulatory Surgery
CTID: NCT05676294
Phase: Phase 2 Status: Recruiting
Date: 2024-10-08