| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 500mg | |||
| Other Sizes |

| 靶点 |
- Dihydrofolate reductase (DHFR) (IC₅₀: 0.03 μM in Plasmodium falciparum)[2]
- 5-HT₃ receptors (Ki: 1.2 μM in human cloned receptors)[3] |
|---|---|
| 体外研究 (In Vitro) |
- 恶性疟原虫DHFR抑制:Proguanil 对恶性疟原虫DHFR的直接抑制IC₅₀为0.03 μM,通过阻断四氢叶酸合成干扰寄生虫DNA复制。活性代谢产物环氯胍的效力比母体药物高10倍[2]
- 5-HT₃受体拮抗:放射性配体结合实验显示,Proguanil 对人5-HT₃受体的竞争性拮抗Ki为1.2 μM。功能实验证实其可抑制5-HT诱导的HEK293细胞钙内流,表现为功能性拮抗[3] - 犬巴贝斯虫生长抑制:体外实验中,Proguanil (2 μM) 与阿托伐醌 (1 μM) 联用使巴贝斯虫血症减少>90%,协同作用指数 (FICI) 为0.4[5] 氯胍的体外抗疟活性较弱(IC50=2.4-19 μM),其功效依赖于其活性代谢物环胍,IC50为0.5-2.5 nM。二氢叶酸还原酶(DHFR)被环胍抑制。在体外观察到氯胍和阿托伐醌之间的协同作用。此外,这两种药物都能有效对抗配子细胞和红细胞前(肝脏)阶段的疟原虫[1]。通过充当双胍而不是其代谢物环胍(一种寄生虫二氢叶酸还原酶 [DHFR] 抑制剂),氯胍增强了阿托伐醌的作用。由于氯胍不会改变其他线粒体电子传递抑制剂(如粘噻唑和抗霉素)的作用,因此氯胍介导的增强作用仅限于阿托伐醌[2]。氯胍、代谢物 4-氯苯基-1-双胍 (CPB) 和活性代谢物环胍 (CG) 可逆地阻断 5-HT3 受体反应,IC50 分别为 1.81、1.48 和 4.36 μM [3]。 |
| 体内研究 (In Vivo) |
- 疟疾预防(小鼠模型):口服Proguanil (10 mg/kg/天) 可在感染伯氏疟原虫前2天开始给药,提供100%保护。保护效应与血浆环氯胍水平>50 ng/mL相关[1]
- 5-HT₃介导的止吐作用:在顺铂诱导的雪貂呕吐模型中,Proguanil (30 mg/kg,口服) 使干呕次数减少65%,效果与昂丹司琼 (1 mg/kg) 相当,且可被5-HT₃激动剂mCPBG逆转[3] - 犬巴贝斯虫治疗:口服Proguanil (5 mg/kg,每日两次) 联合阿托伐醌 (13.3 mg/kg,每日两次) 治疗7天,80%感染犬在7天内清除虫体,28天随访无复发,显著改善血细胞比容并缓解临床症状[5] 在Wistar品系白化大鼠中,氯胍(口服;2.9毫克/公斤体重;每天一次,持续5天和6周)引起轻度退行性变化持续5天,严重退行性变化持续6周。变更[4]。给予氯胍的大鼠血清睾酮水平显着下降[4]。当两只慢性期和三只急性期实验性感染吉本芽孢杆菌的狗服用马拉隆(阿托伐醌和氯胍)时,寄生虫血症减少,临床症状得到改善[5]。 |
| 酶活实验 |
- DHFR活性测定:重组恶性疟原虫DHFR与Proguanil (0.01–10 μM) 和NADPH孵育,加入二氢叶酸启动反应,340 nm分光光度法检测产物生成。IC₅₀通过非线性回归计算,环氯胍效力显著高于母体药物[2]
- 5-HT₃受体结合实验:表达人5-HT₃A受体的HEK293细胞膜蛋白与[³H]GR65630及递增浓度Proguanil (0.1–100 μM) 孵育,10 μM昂丹司琼定义非特异性结合。Ki通过Cheng-Prusoff方程计算[3] |
| 细胞实验 |
- 恶性疟原虫生长抑制:同步化恶性疟原虫培养物经Proguanil (0.01–10 μM) 处理48小时,[³H]次黄嘌呤掺入法评估寄生虫生长。EC₅₀与DHFR抑制数据一致[2]
- 5-HT₃功能实验:转染5-HT₃A受体的HEK293细胞负载Fura-2 AM,检测10 μM 5-HT刺激下的钙流。Proguanil (0.1–10 μM) 使5-HT浓度-反应曲线右移,证实拮抗作用[3] |
| 动物实验 |
- Malaria prophylaxis study: C57BL/6 mice were infected with P. berghei via intraperitoneal injection. Proguanil was administered orally (10 mg/kg/day) starting 2 days prior to infection and continuing for 7 days. Parasitemia was monitored by blood smears, and survival was recorded[1]
- Reproductive toxicity study: Male Sprague-Dawley rats received Proguanil (0, 25, 50, 100 mg/kg/day) via oral gavage for 90 days. Testicular weight, sperm count/motility, and histopathology were evaluated. Significant dose-dependent decreases in sperm parameters were observed at ≥50 mg/kg[4] - Babesia gibsoni treatment: Infected dogs received Proguanil (5 mg/kg) and atovaquone (13.3 mg/kg) orally twice daily for 7 days. Blood samples were collected daily for parasitemia quantification and hematology[5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapidly and well absorbed in humans following oral doses ranging from 50 to 500 mg. Metabolism / Metabolites Variably metabolized in the liver by cytochrome P450 isoenzymes to the active triazine metabolite, cycloguanil. This variable metabolism of proguanil may have profound clinical importance in poor metabolizers such as the Asian and African populations at risk for malaria infection. Prophylaxis with proguanil may not be effective in these persons because they may not achieve adequate therapeutic levels of the active compound, cycloguanil, even after multiple doses. Proguanil has known human metabolites that include Cycloguanil and 4-Chlorophenylbiguanide. Biological Half-Life Approximately 20 hours - Absorption: Oral Proguanil (100 mg) in humans showed Tmax of 2–4 hours, with bioavailability of 70–80%. Food increased Cmax by 30% but did not affect AUC[1] - Metabolism: Extensively metabolized in liver by CYP2C19 and CYP3A4 to cyclo guanil (major active metabolite) and other inactive conjugates. Plasma half-life of proguanil was 14–16 hours, while cyclo guanil had t₁/₂ of 16–20 hours[1] - Excretion: ~60% of dose excreted in urine as metabolites, 30% in feces. Less than 5% unchanged drug detected in urine[1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
The combination of atovaquone and proguanil has been associated with transient and minor serum aminotransferase elevations in a small proportion of patients. More importantly, there have been rare reports of idiosyncratic acute liver injury due in patients on atovaquone/ proguanil but the number of cases has been too few to define a typical clinical course. In one reported case, the onset of injury was after 3 weeks and presentation was with fatigue and jaundice and a cholestatic pattern of serum enzyme elevations. The injury resolved within 2 months of stopping the medication (Case 1). In another case report of chloroquine and proguanil, liver injury arose within days of starting the combination and the pattern of serum enzyme elevations was mixed. In both cases, allergic features were minimal and autoantibodies were not present. In both cases, combination therapy was used and either agent may have been the cause of the injury. Atovaquone and proguanil have also been linked to rare instances of Stevens Johnson syndrome which is often accompanied by mild liver injury or liver enzyme elevations. Likelihood score: E* (unproven but sometimes suspected cause of clinically apparent liver injury). Protein Binding Approximately 75% - Acute toxicity: LD₅₀ in rats was >2000 mg/kg (oral). Clinical signs included sedation and gastrointestinal disturbances[1] - Reproductive toxicity: In male rats, Proguanil (50 mg/kg/day) caused testicular atrophy, decreased spermatogenesis, and increased abnormal sperm morphology after 90 days. These effects were reversible after 4-week washout[4] - Hematological effects: In vitro human lymphocyte studies showed Proguanil (520 ng/mL) induced dose-dependent DNA damage (comet assay tail moment increased by 40%) without affecting viability. Metabolic activation by S9 mix enhanced genotoxicity[8] |
| 参考文献 |
|
| 其他信息 |
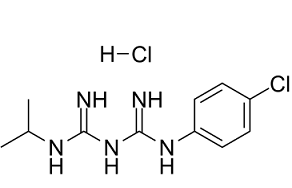
Proguanil Hydrochloride is the hydrochloride salt form of proguanil, a synthetic biguanide derivative of pyrimidine and an folate antagonist with antimalarial property. Upon hydrolysis, proguanil is converted to its active cyclic triazine metabolite, cycloguanil, by a cytochrome P450 dependent reaction. Cycloguanil selectively inhibits the bifunctional dihydrofolate reductase-thymidylate synthase (DHFR-TS) of plasmodium parasite, thereby disrupting deoxythymidylate synthesis and ultimately blocking DNA and protein synthesis in the parasite.
A biguanide compound which metabolizes in the body to form cycloguanil, an anti-malaria agent. See also: Proguanil (annotation moved to). |
| 分子式 |
C11H17CL2N5
|
|---|---|
| 分子量 |
290.19218
|
| 精确质量 |
289.086
|
| 元素分析 |
C, 45.53; H, 5.90; Cl, 24.43; N, 24.13
|
| CAS号 |
637-32-1
|
| 相关CAS号 |
Proguanil;500-92-5;Proguanil-d6 hydrochloride;Proguanil-d6;Proguanil-d4 hydrochloride;1189671-34-8
|
| PubChem CID |
9570076
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
402.7ºC at 760 mmHg
|
| 熔点 |
249-251ºC
|
| 闪点 |
197.4ºC
|
| LogP |
4.065
|
| tPSA |
83.79
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
292
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(C)N=C(N)/N=C(\N)/NC1=CC=C(C=C1)Cl.Cl
|
| InChi Key |
SARMGXPVOFNNNG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C11H16ClN5.ClH/c1-7(2)15-10(13)17-11(14)16-9-5-3-8(12)4-6-9/h3-7H,1-2H3,(H5,13,14,15,16,17)1H
|
| 化学名 |
(1E)-1-[amino-(4-chloroanilino)methylidene]-2-propan-2-ylguanidinehydrochloride
|
| 别名 |
Proguanil HCl; Proguanil hydrochloride; Chloroguanide hydrochloride; Chlorguanide hydrochloride; 637-32-1; Diguanyl; Paludrine; Chloroguanide hydrochloride; Chloroquanil
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4460 mL | 17.2301 mL | 34.4602 mL | |
| 5 mM | 0.6892 mL | 3.4460 mL | 6.8920 mL | |
| 10 mM | 0.3446 mL | 1.7230 mL | 3.4460 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。