| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
奎奴普汀/达福普汀是一种抗生素组合,包括链霉素,用于治疗感染。有报道称奎努普丁/达福普丁对支原体有效。[1]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Quinupristin and dalfopristin is distributed into milk in rats ... . The pharmacokinetics of quinupristin/dalfopristin have been studied in rats, monkeys and humans following intravenous infusion of radiolabelled and unlabelled drug. In rats and monkeys quinupristin and dalfopristin undergo rapid elimination from the blood and wide tissue distribution. Nevertheless, they do not penetrate the central nervous system or cross the placenta to any significant degree and they do not appear to be subject to significant body retention following cessation of administration. The blood elimination half-life of quinupristin was approximately 0.6 hr in rats and 0.5 hr in monkeys, and that of dalfopristin was approximately 0.6 hr and 0.2 hr, respectively. Both compounds are primarily eliminated through the bile into the faeces; quinupristin is mainly excreted unchanged whereas dalfopristin is extensively metabolized beforehand. The metabolites include the microbiologically active pristinamycin PIIA for dalfopristin and the microbiologically active glutathione- and cysteine-conjugated derivatives for quinupristin. Quinupristin and dalfopristin appear to be handled in a similar manner by humans. Following intravenous administration both compounds are rapidly cleared from the blood with elimination half-lives of approximately 1 hr for quinupristin and 0.4-0.5 hr for dalfopristin. The pharmacokinetic profile of quinupristin is dose-independent and so is that of dalfopristin and RP 12536 when considered together. Extravascular diffusion of quinupristin/dalfopristin has been assessed in human non-inflammatory interstitial fluid. Fecal excretion constitutes the main elimination route for both parent drugs and their metabolites (75 to 77% of dose). Urinary excretion accounts for approximately 15% of the quinupristin and 19% of the dalfopristin dose. Preclinical data in rats have demonstrated that approximately 80% of the dose is excreted in the bile and suggest that in man, biliary excretion is probably the principal route for fecal elimination. Metabolism / Metabolites Quinupristin is converted to two conjugated active major metabolites, one with glutathione and one with cysteine. Quinupristin and dalfopristin are converted to several major active metabolites: 2 conjugated (with glutathione and cysteine) metabolites for quinupristin and one nonconjugated (formed by hydrolysis) metabolite for dalfopristin, which also act synergistically with the complementary parent drug. This conversion occurs in vitro by nonenzymatic reactions independent of cytochrome P-450 (CYP) and glutathione transferase enzymes. Biological Half-Life 3.1 hours The elimination half-life of quinupristin and dalfopristin is approximately 0.85 and 0.70 hours, respectively. The pharmacokinetics of quinupristin/dalfopristin have been studied in rats, monkeys and humans following intravenous infusion of radiolabelled and unlabelled drug. ... The blood elimination half-life of quinupristin was approximately 0.6 hr in rats and 0.5 hr in monkeys, and that of dalfopristin was approximately 0.6 hr and 0.2 hr, respectively. ... Following intravenous administration both compounds are rapidly cleared from the blood with elimination half-lives of approximately 1 hr for quinupristin and 0.4-0.5 hr for dalfopristin. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Moderate. |
| 参考文献 |
[1]. Gurk-Turner C. Quinupristin/dalfopristin: the first available macrolide-lincosamide-streptogramin antibiotic. Proc (Bayl Univ Med Cent). 2000 Jan;13(1):83-6.
[2]. Tantibhedhyangkul W, et al. Anti-Mycoplasma Activity of Daptomycin and Its Use for Mycoplasma Elimination in Cell Cultures of Rickettsiae. Antibiotics (Basel). 2019 Aug 21;8(3). |
| 其他信息 |
Quinupristin/dalfopristin is a combination of two antibiotics used to treat infections by staphylococci and by vancomycin-resistant Enterococcus faecium. Dalfopristin inhibits the early phase of protein synthesis in the bacterial ribosome and quinupristin inhibits the late phase of protein synthesis. The combination of the two components acts synergistically and is more effective in vitro than each component alone.
Quinupristin is a Streptogramin Antibacterial. Quinupristin is a semi-synthetic derivative of pristinamycin, a natural occurring type B streptogramin. Quinupristin binds to the bacterial 50S ribosomal subunit, thereby inhibiting peptide chain elongation, and causing early termination of normal bacterial protein synthesis. Quinupristin is primarily effective against gram-positive cocci. Drug Indication For the treatment of bacterial infections (usually in combination with dalfopristin). FDA Label Mechanism of Action Quinupristin inhibits the late phase of protein synthesis in the bacterial ribosome. Dalfopristin binds to the 23S portion of the 50S ribosomal subunit, and changes the conformation it, enhancing the binding of quinupristin by a factor of about 100. In addition, it inhibits peptidyl transferase. Quinupristin binds to a nearby site on the 50S ribosomal subunit and prevents elongation of the polypeptide as well as causing incomplete chains to be released. The site of action of quinupristin and dalfopristin is the bacterial ribosome. Dalfopristin has been shown to inhibit the early phase of protein synthesis while quinupristin inhibits the late phase of protein synthesis. Synercid is bactericidal against isolates of methicillin-susceptible and methicillin-resistant staphylococci. The mode of action of Synercid differs from that of other classes of antibacterial agents such as beta-lactams, aminoglycosides, glycopeptides, quinolones, macrolides, lincosamides and tetracyclines. Therefore, there is no cross resistance between Synercid and these agents when tested by the minimum inhibitory concentration (MIC) method. The unique mechanism of action for quinupristin and dalfopristin is inhibition of the late (peptide chain elongation inhibition) and early (peptidyl transferase inhibition and resultant conformational changes) phases of protein synthesis, respectively, by binding at different sites on the 50S subunit of the bacterial ribosome. Antagonism of beta-lactams, aminoglycosides, glycopeptides, quinolones, macrolides, lincosamides, or tetracyclines has not occurred in vitro. The bacterial ribosome is a primary target of several classes of antibiotics. Investigation of the structure of the ribosomal subunits in complex with different antibiotics can reveal the mode of inhibition of ribosomal protein synthesis. Analysis of the interactions between antibiotics and the ribosome permits investigation of the specific effect of modifications leading to antimicrobial resistances. Streptogramins are unique among the ribosome-targeting antibiotics because they consist of two components, streptogramins A and B, which act synergistically. Each compound alone exhibits a weak bacteriostatic activity, whereas the combination can act bactericidal. The streptogramins A display a prolonged activity that even persists after removal of the drug. However, the mode of activity of the streptogramins has not yet been fully elucidated, despite a plethora of biochemical and structural data. RESULTS: The investigation of the crystal structure of the 50S ribosomal subunit from Deinococcus radiodurans in complex with the clinically relevant streptogramins quinupristin and dalfopristin reveals their unique inhibitory mechanism. Quinupristin, a streptogramin B compound, binds in the ribosomal exit tunnel in a similar manner and position as the macrolides, suggesting a similar inhibitory mechanism, namely blockage of the ribosomal tunnel. Dalfopristin, the corresponding streptogramin A compound, binds close to quinupristin directly within the peptidyl transferase centre affecting both A- and P-site occupation by tRNA molecules. The crystal structure indicates that the synergistic effect derives from direct interaction between both compounds and shared contacts with a single nucleotide, A2062. Upon binding of the streptogramins, the peptidyl transferase centre undergoes a significant conformational transition, which leads to a stable, non-productive orientation of the universally conserved U2585. Mutations of this rRNA base are known to yield dominant lethal phenotypes. It seems, therefore, plausible to conclude that the conformational change within the peptidyl transferase centre is mainly responsible for the bactericidal activity of the streptogramins and the post-antibiotic inhibition of protein synthesis. |
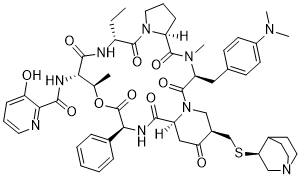
| 分子式 |
C53H67N9O10S
|
|---|---|
| 分子量 |
1022.22
|
| 精确质量 |
1021.47
|
| CAS号 |
120138-50-3
|
| 相关CAS号 |
Quinupristin mesylate
|
| PubChem CID |
5388937
|
| 外观&性状 |
White to slightly yellow powder
White crystals in combination with methanol |
| 密度 |
1.38g/cm3
|
| 熔点 |
approximately 200 °C
|
| 折射率 |
1.662
|
| LogP |
3.123
|
| tPSA |
256.5
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
14
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
73
|
| 分子复杂度/Complexity |
2010
|
| 定义原子立体中心数目 |
9
|
| SMILES |
CC[C@@H]1C(=O)N2CCC[C@H]2C(=O)N([C@H](C(=O)N3C[C@H](C(=O)C[C@H]3C(=O)N[C@H](C(=O)O[C@@H]([C@@H](C(=O)N1)NC(=O)C4=C(C=CC=N4)O)C)C5=CC=CC=C5)CS[C@@H]6CN7CCC6CC7)CC8=CC=C(C=C8)N(C)C)C
|
| InChi Key |
WTHRRGMBUAHGNI-LCYNINFDSA-N
|
| InChi Code |
InChI=1S/C53H67N9O10S/c1-6-37-50(68)61-23-11-14-38(61)51(69)59(5)40(26-32-16-18-36(19-17-32)58(3)4)52(70)62-28-35(30-73-43-29-60-24-20-33(43)21-25-60)42(64)27-39(62)47(65)57-45(34-12-8-7-9-13-34)53(71)72-31(2)44(48(66)55-37)56-49(67)46-41(63)15-10-22-54-46/h7-10,12-13,15-19,22,31,33,35,37-40,43-45,63H,6,11,14,20-21,23-30H2,1-5H3,(H,55,66)(H,56,67)(H,57,65)/t31-,35+,37-,38+,39+,40+,43-,44+,45+/m1/s1
|
| 化学名 |
N-[(3S,6S,12R,15S,16R,19S,22S,25R)-25-[[(3S)-1-azabicyclo[2.2.2]octan-3-yl]sulfanylmethyl]-3-[[4-(dimethylamino)phenyl]methyl]-12-ethyl-4,16-dimethyl-2,5,11,14,18,21,24-heptaoxo-19-phenyl-17-oxa-1,4,10,13,20-pentazatricyclo[20.4.0.06,10]hexacosan-15-yl]-3-hydroxypyridine-2-carboxamide
|
| 别名 |
Antibiotic RP 57669 QuinupristinQuinupristin RP 68888 RP 57669 RP57669RP-68888 RP68888 RP-57669
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~122.28 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.9783 mL | 4.8913 mL | 9.7826 mL | |
| 5 mM | 0.1957 mL | 0.9783 mL | 1.9565 mL | |
| 10 mM | 0.0978 mL | 0.4891 mL | 0.9783 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01371656 | COMPLETEDWITH RESULTS | Drug: levofloxacin | Acute Leukemias of Ambiguous Lineage Bacterial Infection Diarrhea Fungal Infection |
Children's Oncology Group | 2011-09 | Phase 3 |
| NCT00240747 | TERMINATED | Drug: Synercid | Gram-Positive Bacterial Infections | Pfizer | 2000-06 | Phase 3 |
| NCT02099240 | TERMINATED | Drug: oral antibiotics Procedure: intravenous antibiotics |
Osteomyelitis | Julio Ramirez | 2014-03-06 | Early Phase 1 |