| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 靶点 |
Nucleoside analogue; Influenza virus
|
|---|---|
| 体外研究 (In Vitro) |
Triazavirin 对蜱传脑炎病毒的有效性是在敏感的细胞培养物中测量的。 Triazavirin 在 SKEV 细胞培养物中的浓度为 128 mcg/mL,可有效抑制蜱传脑炎病毒(Sofiin 株)的繁殖[2]。
在第一阶段,我们研究了三氮唑核苷钠(Triazavirin sodium,Riamilovir)和乙酰水杨酸对体外血小板聚集的影响。ADP诱导的兔全血对照阻抗为8.3 Ω。100 μM浓度的参比药物将血小板聚集幅度降至3.4 Ω,相当于相对于对照显著抑制了59.8%的血小板功能活性(表1)。将乙酰水杨酸浓度降至10和1 μM后,血小板聚集幅度分别降至5.5和6.6 Ω。因此,在上述浓度下,乙酰水杨酸分别抑制了23.7%和11.6%的血小板聚集。参比药物的IC50为57.5 μM。 100 μM浓度的三氮唑核苷钠(Riamilovir)将ADP诱导的血小板聚集幅度降至6.9 Ω,即抑制了17.1%的聚集过程(表1)。在全血与LPS孵育时,血小板聚集幅度从8.3显著增加至11.9 Ω,表明血小板止血系统在巨噬细胞激活后活性增强(表2)。研究药物的抗血小板活性评估基于ADP诱导的完整血小板与LPS处理血小板聚集水平差异范围。 100 μM浓度的乙酰水杨酸将血小板聚集幅度显著降至8.91 Ω,即抑制了83.2%的聚集过程(表2,图1)。10和1 μM浓度下,乙酰水杨酸分别抑制了52.4%和3.2%的活性,血小板聚集幅度分别降至10.02和11.8 Ω。乙酰水杨酸的IC50值为12.4 μM(表2)。因此,在LPS刺激巨噬细胞后,参比药物的活性比在完整血液中提高了4.6倍。 三氮唑核苷钠(Riamilovir)在LPS存在下显示出高抗血小板活性:100 μM浓度下抑制了96.3%的血小板聚集(图1),并将该过程幅度降至8.43 Ω(表2)。当riamilovir浓度降至10和1 μM时,血小板聚集幅度分别降至9.9和10.9 Ω,即分别抑制了56.1%和26.8%的血小板聚集(图1)。riamilovir的IC50为5.2 μM。因此,体外实验表明,在LPS刺激巨噬细胞的条件下,riamilovir的IC50值比乙酰水杨酸高2.4倍。[4] |
| 体内研究 (In Vivo) |
研究了三氮唑核苷治疗白化小鼠实验性森林泉脑炎的有效性。研究结果表明,高剂量(200-400 mg/kg)的三氮杂韦林可以适度保护受感染的动物。测试组的动物寿命显着延长(从 4.1 天延长至 4.8 天),并且靶器官中病毒积累量显着下降[3]。
第二阶段旨在验证三氮唑核苷钠(Riamilovir)在体内实验是否具有相同效应。对照组大鼠ADP诱导的血小板聚集幅度为7.9Ω。20mg/kg剂量的抗病毒药物Riamilovir使血小板聚集幅度降至5.57Ω,血小板功能活性抑制率达29.4%(表3),证实该药物在体内具有抗血小板作用。 静脉注射LPS的大鼠,其ADP诱导的血小板聚集幅度较空白对照组显著升高(达10.9Ω),表明高细胞因子血症可激活血小板。Riamilovir将血小板聚集幅度降至8.98Ω,在细胞因子中毒条件下的抗血小板活性较正常动物提高2.2倍。[4] 两种治疗方案中,三氮唑核苷钠(Riamilovir)均能缩短住院周期。每日高剂量给药组患者住院时间最短。该药物可减轻疾病全身感染症状的持续时间和严重程度,其中每日1250mg、持续5天给药方案的患者发热总时长和呼吸道综合征持续时间最短,且未记录不良反应。该剂量组在治疗第6天实现100%的急性呼吸道病毒感染病原体清除率。 结论:三氮唑核苷钠(Riamilovir)在两种治疗方案中均表现出临床有效性和良好安全性。每日1250mg给药方案能产生更显著的临床效果,研究组在住院第6天即实现病原体完全清除。[5] TZV/三氮唑病毒(利阿昔洛韦)在动物模型中的抗流感活性。[6] 如表3所示,当根据治疗和预防方案给药时(感染前24和1小时以及感染后24、48和72小时),TZV保护小鼠免受A型和B型流感病毒引起的死亡。TZV以1至200mg/kg体重的剂量范围通过i.g.途径给药。确定最佳有效剂量为50至100mg/kg体重。从数据中可以明显看出,TZV和金刚乙胺对感染血清型a流感病毒(a/Aichi/2/68[H3N2])的小鼠提供了相似水平的保护,但感染B型(B/Lee/40)并以大约相同剂量治疗的动物的存活率是金刚乙胺的三到四倍。因此,TZV可以保护65%至75%的感染A或B病毒的小鼠。当应用TZV给药的治疗(+24小时、+48小时和+72小时)或预防(-24小时和-1小时)方案时,TZV也是有效的(数据未显示)。同样值得注意的是,TZV的毒性较低:小鼠腹腔注射TZV后,LD50为1400±120mg/kg体重,肌肉注射TZV的LD50为2200±96mg/kg体重。潜在抗病毒药物的基本特征是它们的稳定性、代谢转化、药代动力学和生物利用度。 |
| 酶活实验 |
本研究依据现行《新药理学物质临床前研究手册》要求进行。三氮唑核苷钠(Triazavirin sodium,Riamilovir)作为抗病毒药物被选为研究对象。体外实验中以抗血小板药物乙酰水杨酸作为对照药,选择依据是该药物作为循证等级较高的抗血小板剂被广泛应用。
三氮唑核苷钠水合物(Riamilovir)使用生理盐水溶解;对照药先溶于30μl DMSO,再用生理盐水稀释至所需体积。采用Chrono-Log-700双通道发光聚集仪,通过阻抗法检测药物对血小板聚集的影响。
体外实验用兔血经耳缘静脉自由滴落法采集,用3.8%枸橼酸钠(9:1)抗凝。取450μl恒定体积全血用于研究。将100μM浓度的药物直接加入含全血的比色杯,5μM ADP作为血小板聚集诱导剂。
若显示高抗血小板活性,为计算IC50值(抑制50%血小板聚集的浓度),需追加检测10μM和1μM浓度样本。同时在高细胞因子血症条件下分析抗血小板活性:将终浓度20μM的LPS溶液(大肠杆菌O111:B4)与受试药同步加入全血样本比色杯,孵育5分钟后加入血小板聚集诱导剂。[4]
|
| 细胞实验 |
与活性药物利巴韦林相比,在敏感细胞培养物中评估了三唑韦林对蜱传脑炎病毒的疗效。在128 mcg/ml的浓度下,三唑韦灵通过在SKEV细胞培养物内积累而对蜱传乙脑病毒繁殖(Sofin株)具有抑制活性[2]。
CAM模型中抗病毒活性的体外研究[6] 选取11-13日龄鸡胚绒毛尿囊膜(CAM)剪切成约1mm³碎片,悬浮于含青霉素和硫酸链霉素的Hanks盐溶液中。将含病毒的起始尿囊液按10⁻¹至10⁻⁷梯度稀释后加入单层细胞长满的孔板,每孔悬浮液37℃孵育1小时,随后加入不同浓度的三氮唑核苷(Riamilovir)TZV水溶液。参照文献26方法,经36-37℃孵育48小时后,通过血凝素滴度测定和空斑试验评估抗病毒活性。 三氮唑核苷(Riamilovir)在兔肝匀浆中的稳定性[6] 按类似文献方法制备兔肝匀浆,反应体系含25mg/ml蛋白和500μM TZV,37℃孵育。定时取样后立即加入预冷甲醇至66%(v/v)终止反应,10000×g离心4分钟收集沉淀。上清液经SpeedVac冷冻干燥后,残渣水溶解,通过前述HPLC方法分析产物浓度(按峰面积定量)。 TZV/三氮唑核苷(Riamilovir)在细胞培养中的代谢[6] 将TZV水溶液加入含6×10⁶个HEK 293T肾细胞或Huh7肝细胞单层的培养皿,终浓度1mM。细胞与化合物共孵育1.5小时或24小时后,用PBS缓冲液洗涤三次,等体积PBS重悬并通过三次冻融法裂解。加入等体积6%三氟乙酸离心后取上清,用饱和Na₂CO₃调至中性pH,按上述HPLC方法分析代谢产物。 |
| 动物实验 |
The comparative study of the therapeutic efficacy of Triazavirin against experimental Forest-Spring encephalitis on albino mice vs. the active drug Ribavirin® showed that in high doses (200-400 mg/kg) Triazavirin moderately protected the infected animals. A significant increase of the animal lifespan in the test groups (from 4.1 to 4.8 days) and a statistically (p ≤ 0.05) valid decrease of the virus accumulation in the target organ (the brain) were observed[3].
In vivo experiments were performed on rats divided into 4 groups (6 animals per group): two groups without LPS administration (intact rats and rats intragastrically treated with Triazavirin sodium hydrate (Riamilovir)) and two groups with LPS intoxication (control animals received intravenous injection of LPS and experimental rats received LPS intravenously and riamilovir intragastrically). Riamilovir in a dose of 20 mg/kg (a dose equivalent to human dose calculated using interspecies conversion factor) was administered once intragastrically using an atraumatic gastric probe 1 h before blood sampling (the corresponding to maximum concentration in the blood). The rats were anaesthetized with chloral hydrate (400 mg/kg intraperitoneally) and the biomaterial for the study was taken from the abdominal aorta (blood stabilizer indicated above). Hypercytokinemia was modeled by intravenous injection of 2 mg/kg LPS into the caudal vein. Riamilovir was administered orally once 1 h before LPS administration, the blood was taken in 4 h after LPS administration. Controls intragastrically received an equivalent volume of distilled water. The results were processed statistically using GraphPad Prism 8.0 software (one-way ANOVA with Bonferroni correction, p<0.05). IC50, the mean and standard deviation in each group were calculated using Microsoft Excel 2020 built-in functions.[4] Aim: To evaluate the clinical efficacy and safety of antiviral drug Triazavirin sodium hydrate (Riamilovir) in patients with acute respiratory viral infections (ARVI) of non-coronavirus (SARS-CoV-2) etiology with different dosing regimens. Materials and methods: The study included 150 patients with ARVI aged 18-27 years (50 patients received Triazavirin sodium hydrate (Riamilovir) in the regimen of 250 mg 3 times a day for 5 days, 50 patients received riamilovir in the off label regimen of 250 mg 5 times a day for 5 days, 50 patients received only pathogenetic treatment).[5] Anti-influenza activity of Triazavirin (Riamilovir)TZV in animal models. [6] Antiviral activity in CBA mice infected with the A/Aichi/2/68 (H3N2) or B/Lee/40 influenza virus was assessed. Each group of mice included 20 animals. The virus was administered intranasally at 1 and 10 50% lethal doses (LD50) under slight ether anesthesia. A TZV water solution (0.2 ml) was administered by the intragastric (i.g.) route according to one of three schemes: the treatment-and-prophylactic scheme (24 and 1 h before infection [−24 and −1 h] and 24, 48, and 72 h after infection [+24, +48, and +72 h]), the prophylactic scheme (−24 h and −1 h), or the treatment scheme (+24 h, +48 h, and +72 h). Rimantadine was used as a control. The animals were watched for 14 days, and deaths in the control and experimental groups were reported every day. Based on these data, the degree of animal protection was calculated for TZV and compared with that of rimantadine. Pharmacokinetics in rabbits. [6] For a single i.g. administration of Triazavirin (Riamilovir)/TZV to rabbits, the animals (n = 4) were anesthetized with a 10:1 ether-Fluorothane mixture, and a polyurethane gastrointestinal tube was introduced 15 cm deep. Triazavirin (Riamilovir)/TZV was administered as a water solution (12 ml) at a dose of 105 mg/kg of body weight. For i.v. administration to rabbits (n = 4), TZV (4.3 mg/kg of body weight) was injected into the vena auricularis marginalis in physiological solution (1 ml) for 1 min according to the method of reference 11. At predetermined time points up to 24 h postdosing, blood samples (1 ml on average) were collected from the vena auricularis marginalis in a self-flowing manner into microtubes containing 5 μl of heparin (5,000 U/ml). The tubes were shaken, and 0.5-ml aliquots were taken out, mixed with methanol (1 ml), and stored at −24°C. The control blood samples were taken before administration of the test compounds. Prior to HPLC analysis, the samples were centrifuged at 1,500 × g for 10 min; the supernatant was evaporated in a vacuum; and the residue was dissolved in water (100 μl). The samples were then analyzed by HPLC under the conditions described above. Pharmacokinetic parameters were calculated using the Kinetica program. Pharmacokinetics following i.g. administration was studied by the extravascular noncompartmental model of the Thermo Kinetica program. For i.v. administration, the noncompartmental i.v. infusion model was used. The following parameters were determined: the total area under the plasma concentration-versus-time curve (AUCtot), the apparent elimination half-life (T1/2), the maximum concentration of the compound in plasma (Cmax), the time to Cmax (Tmax), and the mean residence time (MRT). The i.g. bioavailability (F) of the compound was calculated as (AUCi.g./dosei.g.)/(AUCi.v./dosei.v.), where AUCi.g. is the AUC of the compound after i.g. administration, dosei.g is the i.g. dose, AUCi.v is the AUC after i.v. administration, and dosei.v. is the i.v. dose. Total clearance (CL) from plasma was calculated as dose/AUC. The volume of distribution (Vss) of TZV at steady state was determined as CL × MRT. |
| 药代性质 (ADME/PK) |
TZV/Triazavirin (Riamilovir) pharmacokinetic parameters following single-dose i.v. or i.g. administration to rabbits. [6]
To determine the i.g. bioavailability of TZV, the compound was administered to rabbits (n = 4) by the i.g. and i.v. routes. At predetermined time points up to 24 h postdosing, blood samples were collected and analyzed by HPLC. For 10 min after i.g. administration, the TZV peak, with a retention time (Tret) of 22.5 min, was the only one observed in rabbit blood, whereas 2 h later, a new peak (M1) appeared, increasing with time, with a Tret of 27.5 min. The concentrations of TZV and M1 in rabbit blood within 12 h after dosing (105 mg/kg of body weight) are shown in Fig. 2A. The Cmax of TZV (1.1 mg/liter) was achieved in 0.40 ± 0.16 h, and the half-time of elimination was 1.1 h. The CL of TZV was 37.0 ± 11.2 liters/h·kg, and the Vss was 83.5 ± 19.2 liters/kg. The M1 concentration increased for 3 h and then decreased insignificantly over the next 5 h. Following i.v. administration of a 4.3-mg/kg dose of TZV to rabbits, the concentration of TZV in plasma fell quickly, with a T1/2 of 0.9 h (Fig. 2B). In contrast to the findings for i.g. administration, the metabolite M1 was not detected. The only product observed during the whole experimental time (24 h) was TZV. A summary of the values of the pharmacokinetic parameters of TZV and its metabolite is provided in Table 4. The i.g. bioavailability (F) of TZV in rabbits was calculated as 12.5%. Formation of the TZV/Triazavirin (Riamilovir) metabolite (M1) in a rabbit liver homogenate. [6] Most likely the TZV metabolite was formed in the liver or kidney. To confirm this assumption, TZV was incubated with a rabbit liver homogenate. Figure 3 shows the HPLC analysis of the liver homogenate after incubation with 500 μM TZV. As can be observed, a new peak, increasing with time, was observed after 10 min of incubation. The retention time of this peak agreed well with that of the metabolite formed in rabbit blood following i.g. administration of TZV. TZV/Triazavirin (Riamilovir) metabolism in HEK 293T kidney and Huh7 liver cell cultures. [6] HEK 293T or Huh7 cell cultures were incubated with 500 μM TZV for different times. HPLC analysis of cellular extracts revealed the presence of both TZV and the metabolite, whose retention time agreed with those of the M1 metabolite formed in a rabbit liver homogenate and the metabolite detected in rabbit blood following i.g. TZV administration (Fig. 4). TZV/Triazavirin (Riamilovir) metabolite structure. [6] The identities of the metabolites found in cell cultures and in rabbit blood following i.g. administration and upon incubation of TZV in a rabbit liver homogenate were confirmed by comparison of retention times using data from HPLC analysis, UV spectra, and mass spectra. The patterns of UV spectra for TZV and the metabolite formed upon incubation of TZV with a rabbit liver homogenate are shown in Fig. 5. The patterns for TZV and M1 looked obviously different: TZV had two λmax, one at 257 nm and one at 360 nm, while the λmax for M1 were at 249 nm and 320 nm. We assumed that the TZV nitro group could be reduced to give 2-methylthio-6-amino-1,2,4-triazolo[5,1-c]-1,2,4-triazine-7(4H)-one (AMTZV). AMTZV was synthesized, and we showed that the UV pattern of AMTZV was identical to that of M1. For verification of the structure of the M1 metabolite formed in the liver homogenate, its mass spectrum was compared with that of the synthesized compound (Fig. 6A and B). A major ion was [M-H]−, with a molecular mass of 197, which agrees with that of AMTZV. Also, a peak of the TZV [M-H]− ion 227 was present. For ions 197 and 227, the respective satellite ions corresponding to 198 [M + 1-H]− and 199 [M + 2-H]−, as well as 228 [M + 1-H]− and 229 [M + 1-H]−, were observed. The ion structure corresponding to peak 213 is obscure. Peak 167 may relate to a metabolite degradation product, which was confirmed by mixing [M1 + AMTZV] and [M1 + 15N-AMTZV] (data not shown). |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Data regarding the protein binding of triazavirin is not readily available. |
| 参考文献 |
|
| 其他信息 |
Novel method for the coating of positively charged liposomes with modified chitosan was elaborated. Liposomes were prepared by stepwise extrusion through inorganic membranes (Anotop) of 0.2 and 0.1 μm pore sizes. Chitosan derivatives were synthesized via the Ugi multicomponent reaction. Several series of liposomal compositions were produced and their properties were compared in terms of particle size, polydispersity index (PDI), zeta potential and stability. The effect of various additives was investigated and the optimal composition of the lipid film was determined. The addition of the uncharged fatty esters allowed the diameter of the liposomes obtained by extrusion to be reduced to 145-150 nm with a PDI of 0.13-0.15. The prepared liposomes were loaded with the novel antiviral drug Triazavirin and used to determine the release profile. Triazavirin was included into liposome layer as a salt with biocompatible choline derivatives of limiting fatty acids. The appropriate lipid composition was used for the preparation of a larger quantity of liposomes coated by modified chitosan. It was shown that an appropriate combination of liposomes and polysaccharide layer potentially extended colloidal stability by up to 3 months and exhibited broad functional capabilities for surface modification. [1]
The efficacy of Triazavirin against the tick-borne encephalitis virus was estimated in the sensitive cell culture vs. the active drug Ribavirin. In a concentration of 128 mcg/ml Triazavirin was shown active in inhibition of the tick-borne encephalitis virus reproduction (strain Sofiin) by accumulation in the SKEV cell culture.[2] The efficacy of Triazavirin against the tick-borne encephalitis virus was estimated in the sensitive cell culture vs. the active drug Ribavirin. In a concentration of 128 mcg/ml Triazavirin was shown active in inhibition of the tick-borne encephalitis virus reproduction (strain Sofiin) by accumulation in the SKEV cell culture. [3] We studied the effect of antiviral agent riamilovir on ADP-induced platelet aggregation in the absence and presence of LPS. Unlike acetylsalicylic acid (reference drug), riamilovir did not exhibit antiplatelet effect in vitro. However, it markedly suppressed platelet reactivity in LPS-treated blood samples and was 2.2-fold superior to acetylsalicylic acid in terms of IC50 value. In in vivo experiments, riamilovir under conditions of hypercytokinemia blocked platelet aggregation in rats by 64%.[4] Influenza viruses of types A and B cause periodic pandemics in the human population. The antiviral drugs approved to combat influenza virus infections are currently limited. We have investigated an effective novel inhibitor of human influenza A and B viruses, triazavirine [2-methylthio-6-nitro-1,2,4-triazolo[5,1-c]-1,2,4-triazine-7(4I)-one] (TZV). TZV suppressed the replication of influenza virus in cell culture and in chicken chorioallantoic membranes, and it protected mice from death caused by type A and B influenza viruses. TZV was also effective against a rimantadine-resistant influenza virus strain and against avian influenza A virus H5N1 strains. The pharmacokinetic parameters and bioavailability of TZV were calculated after the administration of TZV to rabbits. The TZV metabolite AMTZV [2-methylthio-6-amino-1,2,4-triazolo[5,1-s]-1,2,4-triazin(e)-7(4I)-one] was discovered in IAK 293T and Huh7 cell cultures, a liver homogenate, and rabbit blood after intragastric administration of TZV. AMTZV was nontoxic and inactive as an inhibitor of influenza virus in cell culture. Most likely, this metabolite is a product of TZV elimination.[6] |
| 分子式 |
C5H5N6NAO3S
|
|---|---|
| 分子量 |
252.186368703842
|
| 精确质量 |
249.989
|
| 元素分析 |
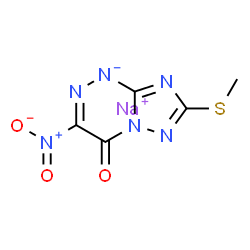
sodium;7-methylsulfanyl-3-nitro-[1,2,4]triazolo[5,1-c][1,2,4]triazin-4-olate
|
| CAS号 |
116061-59-7
|
| 相关CAS号 |
116061-59-7 (sodium);123606-06-4 (free);928659-17-0 (sodium hydrate);
|
| PubChem CID |
46848011
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
144.16
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
16
|
| 分子复杂度/Complexity |
262
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C1C(=NNC2=NC(SC)=NN12)N(=O)=O.[NaH]
|
| InChi Key |
HFCTECILQWPDR-UHFFFAOYSA-M
|
| InChi Code |
InChI=1S/C5H4N6O3S.Na/c1-15-5-6-4-8-7-2(11(13)14)3(12)10(4)9-5;/h12H,1H3;/q;+1/p-1
|
| 化学名 |
sodium;7-methylsulfanyl-3-nitro-[1,2,4]triazolo[5,1-c][1,2,4]triazin-4-olate
|
| 别名 |
Riamilovir sodium; 0HR3OK9WNB; 116061-59-7; (1,2,4)Triazolo(5,1-C)(1,2,4)triazin-4(1H)-one, 7-(methylthio)-3-nitro-, sodium salt; (1,2,4)Triazolo(5,1-C)(1,2,4)triazin-4(6H)-one, 7-(methylthio)-3-nitro-, sodium salt; Sodium 7-(methylthio)-3-nitro-4-oxo-4H-[1,2,4]triazolo[5,1-c][1,2,4]triazin-8-ide; UNII-0HR3OK9WNB
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9653 mL | 19.8263 mL | 39.6526 mL | |
| 5 mM | 0.7931 mL | 3.9653 mL | 7.9305 mL | |
| 10 mM | 0.3965 mL | 1.9826 mL | 3.9653 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。