| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
5-HT1 receptor
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Rizatriptan Benzoate(也称为 MK-462 Benzoate)是一种新型、有效、选择性的血清素 5-HT1B 和 5-HT1D 受体激动剂,可潜在用于治疗急性偏头痛发作。
|
| 体内研究 (In Vivo) |
利扎曲普坦通过作用于血管周围三叉神经上的 5-HT(1D) 受体来阻断神经源性血管舒张,从而抑制麻醉豚鼠体内 CGRP 的释放。利扎曲普坦会引起麻醉豚鼠硬脑膜血管直径的短暂减小,并在 10 分钟内恢复到基线值。利扎曲普坦显着抑制三叉神经节高强度电刺激产生的硬脑膜血浆蛋白外渗。利扎曲普坦显着减少麻醉大鼠的电刺激硬脑膜血管舒张。利扎曲普坦苯甲酸盐显着降低正常组和模型组大鼠中脑SP mRNA水平,表明利扎曲普坦苯甲酸盐可以下调大鼠中脑SP基因表达。 Rizatriptan Benzoate 显着降低偏头痛大鼠模型中脑 PENK mRNA 表达,降低中脑甲硫脑啡肽和亮氨酸脑啡肽水平,从而减弱内源性疼痛调节系统的镇痛作用。[5]
这些研究调查了麻醉豚鼠神经源性硬脑膜血管舒张的药理学。在引入封闭的颅窗后,使用活体显微镜观察脑膜(硬脑膜)血管,并使用视频尺寸分析仪不断测量直径。在用降钙素基因相关肽(CGRP;1微克千克(-1),静脉注射)或硬脑膜局部电刺激(高达300微安)扩张硬脑膜血管之前,用内皮素-1(3微克千克(1),静脉内注射)收缩硬脑膜血管。在用CGRP受体拮抗剂CGRP((8-37))(0.3mg kg(-1),静脉注射)预处理的豚鼠中,电刺激的扩张器反应被抑制了85%,表明CGRP在该物种的神经源性硬脑膜血管舒张中起着重要作用。5-羟色胺(1B/1D)激动剂利扎曲普坦(100微克kg(-1))也阻断了神经源性硬脑膜血管舒张,估计血浆水平与患者抗偏头痛疗效所需的浓度相当Rizatriptan不能逆转CGRP诱发的硬脑膜扩张,表明其对位于支配硬脑膜血管的三叉神经感觉纤维上的突触前受体有作用。此外,选择性5-羟色胺(1D)激动剂PNU-142633(100微克千克(-1))也阻断了神经源性硬脑膜血管舒张,但5-羟色胺(1F)激动剂LY334370(3毫克千克(-1。这种机制可能是利扎曲普坦所例示的曲坦类抗偏头痛作用之一的基础,并表明豚鼠是研究神经源性硬脑膜血管舒张药理学的合适物种。1. 这些对麻醉大鼠的研究表明,使用活体显微镜观察,新型抗偏头痛药物利扎曲普坦显著减少了电刺激的硬脑膜血管舒张,但对外源性P物质或降钙素基因相关肽(CGRP)产生的硬脑膜血管直径的增加没有影响。里扎曲普坦还显著抑制了三叉神经节高强度电刺激产生的硬脑膜血浆蛋白外渗。我们认为利扎曲普坦抑制血管周围三叉神经释放感觉神经肽,以防止神经源性血管舒张和硬脑膜外渗。这些结合前抑制作用可能与利扎曲普坦的抗偏头痛作用有关。2. 本研究利用硝酸甘油诱导的偏头痛大鼠模型,通过实时定量聚合酶链反应检测苯甲酸Rizatriptan对中脑前脑啡肽和P物质基因表达的影响,并研究苯甲酸利扎曲普坦是否可以调节内源性疼痛调节系统。结果表明,苯甲酸利扎曲普坦显著降低了前脑啡肽和P物质的mRNA表达。苯甲酸利扎曲普坦可能抑制内源性疼痛调节系统的镇痛作用。3. 通过免疫组织化学染色,对清醒大鼠上矢状窦周围硬脑膜电刺激后大脑中Fos的表达进行了系统研究。Fos样免疫反应神经元主要分布在上颈脊髓、三叉神经脊髓尾侧核、中缝大核、中脑导水管周围灰质、下丘脑腹内侧核和丘脑中。经腹腔注射苯甲酸Rizatriptan预处理后,三叉神经脊束核尾侧和中缝大核中Fos样免疫反应神经元的数量减少,中脑导水管周围灰质中Fos类免疫反应神经元数量增加,下丘脑腹内侧核和丘脑中侧核保持不变。这些结果提供了形态学证据,表明上述细胞核参与了三叉神经血管性头痛的发展和维持。[4] |
| 酶活实验 |
SYBR绿色实时定量PCR[3]
20微升反应包括10μL SYBR Premix Ex Taq™、0.4μL上游和下游引物(10μM)、0.4μL ROX参考染料、2.0μL cDNA和6.8μL dH2O。通过定量PCR处理不同浓度的质粒标准样品(1.2×103−1.2×109)拷贝/μL。每个样本均一式三份。反应条件如下:94°C预变性2分钟,94°C变性30秒,62°C退火30秒,72°C延伸30秒,共40个循环。在每个周期的退火结束时测量荧光信号,在PCR扩增过程中定义测量临界点,即与荧光信号进入背景水平以上指数增长阶段的拐点相对应的阈值周期值。以95°C持续15秒、60°C持续20秒和95°C连续15秒的模式进行熔解曲线分析。 |
| 动物实验 |
In preliminary experiments it was found that, following introduction of the cranial window, the dural blood vessels typically were observed to be maximally dilated, so that electrical stimulation of the cranial window produced little if any increase in diameter. It was therefore necessary to preconstrict the dural vessels with intravenously administered endothelin-1 (ET-1, 3 μg kg−1) which produced an approximate 50% reduction in dural blood vessel diameter (unpublished observations). Following administration of endothelin-1 (3 μg kg−1, i.v.) dural vasodilation was reliably evoked approximately 3 min later by intravenous rat-αCGRP (1 μg kg−1) or electrical stimulation of the cranial window (250–300 μA, 5-Hz, 1 ms for 10 s) and expressed as percentage increase in dural blood vessel diameter±s.e.mean from baseline. Rizatriptan benzoate (0.01–1 mg kg−1), PNU142,633 (0.01–1 mg kg−1) or LY334370 (3 mg kg−1) were administered intravenously 12 min before administration of ET-1 whereas human-αCGRP(8–37) (0.3 mg kg−1) was given 2 min prior to ET-1. Statistical comparisons between drug and vehicle treated rats were made by t-tests (BMDP statistical software) and P<0.05 was considered significant. [1]
Migraine model establishment and interventions [3] Rizatriptan benzoate control and treatment groups were intragastrically perfused with rizatriptan benzoate, 1 mg/kg per day (according to the adult daily dose), and normal control and model groups were perfused with normal saline 2 mL per day. After 7 days, nitroglycerin (10 mg/kg) was subcutaneously injected into the buttocks of the rizatriptan benzoate treatment and model groups to induce migraine. Normal saline (2 mL/kg) was injected into the normal control and rizatriptan benzoate control groups. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rizatriptan is readily absorbed (approximately 90%) following oral administration; however, the mean oral absolute bioavailability of the rizatriptan tablet is about 45%, owing to extensive first-pass metabolism. The Tmax is approximately one to 1.5 hours. The presence of a migraine headache did not appear to affect the absorption or pharmacokinetics of rizatriptan. Food has no significant effect on the bioavailability of rizatriptan but delays the time to reach peak concentration by an hour. In clinical trials, rizatriptan was administered without regard to food. The bioavailability and Cmax of rizatriptan were similar following the administration of rizatriptan tablets and rizatriptan orally disintegrating tablets. Still, the absorption rate is somewhat slower with orally disintegrating tablets, with Tmax delayed by up to 0.7 hours. The AUC of rizatriptan is approximately 30% higher in females than males. No accumulation occurred on multiple dosing. Following oral administration of a single 10 mg of 14C-rizatriptan, the total radioactivity of the administered dose recovered over 120 hours in urine and feces was 82% and 12%, respectively. Following oral administration of 14C-rizatriptan, rizatriptan accounted for about 17% of circulating plasma radioactivity. Approximately 14% of an oral dose is excreted in urine as unchanged rizatriptan, while 51% is excreted as indole acetic acid metabolite, indicating substantial first-pass metabolism. The mean volume of distribution is approximately 140 L in male subjects and 110 L in female subjects. An early study involving healthy subject reported plasma clearance of 1042 mL/min in males and 821 mL/min in females; however, this difference in clearance rates is not thought to be clinically relevant. Metabolism / Metabolites Rizatriptan primarily undergoes oxidative deamination mediated by monoamine oxidase-A (MAO-A) to form triazolomethyl-indole-3-acetic acid, which is not pharmacologically active. N-monodesmethyl-rizatriptan is a minor metabolite with a pharmacological activity comparable to the parent compound's. Plasma concentrations of N-monodesmethyl-rizatriptan are approximately 14% of those of the parent compound, which is eliminated at a similar rate. Other pharmacologically inactive minor metabolites include the N-oxide, the 6-hydroxy compound, and the sulfate conjugate of the 6-hydroxy metabolite. Rizatriptan is metabolized by monoamine oxidase A isoenzyme (MAO-A) to an inactive indole acetic acid metabolite. In addition, several other inactive metabolites are formed. An active metabolite, N-monodesmethyl-rizatriptan, with pharmacological activity similar to that of the parent compound has been identified in small concentrations (14%) in the plasma. Route of Elimination: Approximately 14% of an oral dose is excreted in urine as unchanged rizatriptan while 51% is excreted as indole acetic acid metabolite, indicating substantial first pass metabolism. Half Life: 2-3 hours Biological Half-Life The plasma half-life of rizatriptan in males and females ranges from two to three hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Breastmilk levels of rizatriptan are low and the half-life in milk is relatively short. Amounts ingested by the infant are small and unlikely to affect the nursing infant. Painful, burning nipples and breast pain have been reported after doses of sumatriptan and other triptans. This has occasionally been accompanied by a decrease in milk production. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk A review of four European adverse reaction databases found 26 reported cases of, painful, burning nipples, painful breasts, breast engorgement and/or painful milk ejection in women who took a triptan while nursing. Pain was sometimes intense and occasionally led to decreased milk production. Pain generally subsided with time as the drug was eliminated. The authors proposed that triptans may cause vasoconstriction of the arteries in the breast, nipples, and the arteries surrounding the alveoli and milk ducts, causing a painful sensation and a painful milk ejection reflex. Protein Binding Rizatriptan is minimally bound (14%) to plasma proteins. |
| 参考文献 | |
| 其他信息 |
Rizatriptan is a member of tryptamines. It has a role as a serotonergic agonist, a vasoconstrictor agent and an anti-inflammatory drug. It is functionally related to a N,N-dimethyltryptamine.
Rizatriptan is a second-generation triptan and a selective 5-HT1B and 5-HT1D receptor agonist. Used in the treatment of migraines, rizatriptan was first approved in the US in 1998. Rizatriptan is available in oral tablets, orally disintegrating tablets (wafers), and oral film formulations. Rizatriptan is a Serotonin-1b and Serotonin-1d Receptor Agonist. The mechanism of action of rizatriptan is as a Serotonin 1b Receptor Agonist, and Serotonin 1d Receptor Agonist. Rizatriptan is only found in individuals that have used or taken this drug. It is a triptan drug used for the treatment of migraine headaches. It is a selective 5-hydroxytryptamine1 receptor subtype agonist.Three distinct pharmacological actions have been implicated in the antimigraine effect of the triptans: (1) stimulation of presynaptic 5-HT1D receptors, which serves to inhibit both dural vasodilation and inflammation; (2) direct inhibition of trigeminal nuclei cell excitability via 5-HT1B/1D receptor agonism in the brainstem and (3) vasoconstriction of meningeal, dural, cerebral or pial vessels as a result of vascular 5-HT1B receptor agonism. See also: Rizatriptan Benzoate (has salt form); Rizatriptan Sulfate (has salt form). Drug Indication Rizatriptan is indicated for the acute treatment of diagnosed migraine with or without aura. Rizatriptan is not indicated for the prophylactic therapy of migraine nor the treatment of cluster headache. In Canada, rizatriptan is approved in adults. In the US, the oral tablet formulations are used in patients six years of age and older and the oral film formation is approved for patients 12 years of age and older weighing 40 kg or more. Treatment of migraine Mechanism of Action There are several physiological and molecular processes implicated in the pathophysiology of migraine. Vasodilation of intracranial extracerebral blood vessels, particularly those supplying the dura mater, has been associated with migraine pain. Activation of the trigeminovascular system leads to the release of vasoactive neuropeptides (such as substance P, calcitonin gene-related peptide (CGRP), and neurokinin A) from the trigeminal nerve innervating the intracranial vessels and dura mater. Vasoactive neuropeptides cause perivascular inflammation and vasodilation in the periphery. Migraine-associated nausea and vomiting are thought to arise from the activation of central and nociceptive sensory neurons that project to autonomic brain-stem nuclei and higher subcortical and cortical pain processing centres. An imbalance in serotonin (5-HT) levels has also been documented: 5-HT binds to 5-HT1B and 5-HT1D receptors to promote trigeminal neuronal firing and vasoconstriction. Rizatriptan is a selective agonist at the 5-HT1B and 5-HT1D receptors on intracranial blood vessels and sensory nerves of the trigeminal system. It binds to these receptors with high affinity. The exact mechanism of action of rizatriptan has not been fully elucidated; however, several documented pharmacological actions of rizatriptan may contribute to its antimigraine effects. Rizatriptan causes vasoconstriction of intracranial extracerebral blood vessels, which is thought to occur primarily via 5-HT1B receptors. Rizatriptan also inhibits nociceptive neurotransmission in trigeminal pain pathways. It attenuates the release of vasoactive neuropeptides by the trigeminal nerve, which is thought to occur via neurogenic and central 5-HT1D receptors. Rizatriptan inhibited neurogenic dural vasodilation and plasma protein extravasation in animal studies. Rizatriptan benzoate is a member of tryptamines. Rizatriptan Benzoate is the benzoate salt form of rizatriptan, a member of the triptan class agents with anti-migraine property. Rizatriptan benzoate selectively binds to and activates serotonin (5-HT) 1B receptors expressed in intracranial arteries, and to 5-HT 1D receptors located on peripheral trigeminal sensory nerve terminals in the meninges and central terminals in brain stem sensory nuclei. Receptor binding results in constriction of cranial vessels and inhibition of nociceptive transmission, thereby providing relief of migraine headaches. Rizatriptan benzoate may also relief migraine headaches by inhibition of pro-inflammatory neuropeptide release. See also: Rizatriptan (has active moiety). The present studies have demonstrated that electrical stimulation of the dura mater evokes neurogenic vasodilation of preconstricted dural blood vessels in anaesthetized guinea-pigs and that the dilation is mediated by CGRP release from trigeminal fibres. In addition neurogenic, but not CGRP-evoked dural vasodilation, was also blocked by rizatriptan at clinically relevant doses via an action on presynaptic 5-HT1D receptors, since neurogenic dural vasodilation was also blocked by the 5-HT1D agonist PNU142,633 but not by the 5-HT1F agonist LY334370. The present studies suggest that the guinea-pig may be an appropriate species in which to investigate the pharmacology of neurogenic dural vasodilation providing data that can be extrapolated to man.[1] Opioid peptides and opioid receptor agonists exert strong analgesic effects by inhibiting neuronal pain-evoked discharges and activating the pain modulatory descending inhibitory system. Enkephalin is classified into two forms according to its structure: met-enkephalin and leu-enkephalin. They are derived from a single precursor, namely, PENK. The results of the present study revealed no significant difference in midbrain PENK expression levels between model and normal control groups, indicating that migraine does not directly influence midbrain PENK expression. However, the effects of migraine on opioid peptide expression require further study. Rizatriptan benzoate significantly reduced midbrain PENK mRNA expression, decreasing the levels of midbrain met-enkephalin and leu-enkephalin, and thereby weakening the analgesic effects of the endogenous pain modulatory system. In addition, SP has been shown to stimulate enkephalin release from the periaqueductal gray. In the present study, rizatriptan benzoate reduced SP and PENK mRNA expression in the midbrain. However, whether there is a correlation between these two reductions remains to be fully investigated. In conclusion, rizatriptan benzoate decreased expression of the mRNAs for SP and PENK in the midbrain, possibly inhibiting the analgesic effects of the endogenous pain modulatory system.[3] |
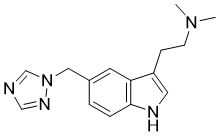
| 分子式 |
C15H19N5
|
|---|---|
| 分子量 |
269.35
|
| 精确质量 |
269.164
|
| 元素分析 |
C, 66.89; H, 7.11; N, 26.00
|
| CAS号 |
144034-80-0
|
| 相关CAS号 |
Rizatriptan-d6 benzoate; 1216984-85-8; 144034-80-0; 159776-67-7 (sulfate)
|
| PubChem CID |
5078
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.21g/cm3
|
| 沸点 |
504.8ºC at 760mmHg
|
| 熔点 |
178-180ºC
|
| 闪点 |
259.1ºC
|
| 蒸汽压 |
2.58E-10mmHg at 25°C
|
| LogP |
1.911
|
| tPSA |
49.74
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
20
|
| 分子复杂度/Complexity |
309
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CN(C)CCC1=CNC2=C1C=C(C=C2)CN3C=NC=N3
|
| InChi Key |
ULFRLSNUDGIQQP-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C15H19N5/c1-19(2)6-5-13-8-17-15-4-3-12(7-14(13)15)9-20-11-16-10-18-20/h3-4,7-8,10-11,17H,5-6,9H2,1-2H3
|
| 化学名 |
N,N-dimethyl-2-[5-(1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]ethanamine
|
| 别名 |
MK-462; MK 462; 144034-80-0; MK 462 free base; 2-(5-((1H-1,2,4-Triazol-1-yl)methyl)-1H-indol-3-yl)-N,N-dimethylethanamine; rizatriptanum; 1H-Indole-3-ethanamine, N,N-dimethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-; N,N-dimethyl-2-[5-(1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]ethanamine; Risatriptan; Rizatriptan
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7126 mL | 18.5632 mL | 37.1264 mL | |
| 5 mM | 0.7425 mL | 3.7126 mL | 7.4253 mL | |
| 10 mM | 0.3713 mL | 1.8563 mL | 3.7126 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04384367 | Recruiting | Drug: Maxalt 10Mg Tablet Drug: Flanax 550mg |
Migraine | Eurofarma Laboratorios S.A. | December 30, 2022 | Phase 3 |
| NCT00897949 | Completed | Drug: rizatriptan benzoate (MK0462) Drug: Comparator: placebo |
Migraine Headache | Organon and Co | March 1995 | Phase 3 |
| NCT00899379 | Completed | Drug: rizatriptan benzoate Drug: Comparator: Placebo |
Migraine Headache | Organon and Co | April 1995 | Phase 3 |
| NCT01286207 | Completed | Drug: Rizatriptan 5 mg Drug: Rizatriptan 10 mg |
Migraine Disorders | Organon and Co | March 1995 | Phase 3 |
| NCT00812006 | Completed | Drug: rizatriptan benzoate Drug: Comparator: placebo |
Migraine | Organon and Co | March 24, 2009 | Phase 3 |