| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500μg |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
GLP-1 receptor
|
|---|---|
| 体外研究 (In Vitro) |
与人 GLP-1(Aib8、Arg34)相比,索马鲁肽有两个氨基酸取代,并且在赖氨酸 26 处衍生化。索马鲁肽的 GLP-1R 亲和力为 0.38±0.06 nM[1]。 Semaglutide 是一种 GLP-1 类似物,与人 GLP-1 具有 94% 的序列同源性[3]。
|
| 体内研究 (In Vivo) |
小型猪静脉注射后索马鲁肽的血浆半衰期为 46 小时,小型猪皮下给药后索马鲁肽的 MRT 为 63.6 小时[1]。 Semaglutide 可改善 1-甲基-4-苯基-1,2,3,6-四氢吡啶 (MPTP) 引起的运动障碍。此外,索马鲁肽还可以挽救酪氨酸羟化酶(TH)水平的降低,减轻炎症反应,减少脂质过氧化,抑制细胞凋亡途径,并增加自噬相关蛋白的表达,以保护黑质和纹状体的多巴胺能神经元。此外,长效GLP-1类似物索马鲁肽在大多数参数上均优于NN-2211[2]。
|
| 酶活实验 |
HEK293‐SNAP‐GLP‐1R细胞在悬浮液中用SNAP‐Lumi4‐Tb(40 nM,Cisbio,Codelet,France)在室温下在完全培养基中培养1小时。在含有0.1%牛血清白蛋白和代谢抑制剂(20 mmol/L 2-脱氧葡萄糖和10 mmol/L NaN3)以防止GLP‐1R内化,如前所述,使用exendin(9‐39)和异硫氰酸荧光素(FITC)安装在K12位置,通过时间分辨förster共振能量转移(FRET)进行结合实验。[4]
|
| 细胞实验 |
Semagulide激活胰腺β细胞中的GLP-1受体,导致葡萄糖依赖性胰岛素释放。它还能减少胰高血糖素的分泌,减缓胃排空,促进饱腹感。
|
| 动物实验 |
Mice: Male C57BL/6 mice 10 weeks old (20-25 g) are used throughout the study. Six groups of mice are randomly assigned (n = 12 per group). The treatments were as follows: (i) saline alone was given to the control group; (ii) NN-2211 group received saline and NN-2211 (25 nmol/kg ip. once daily for 7 days); (iii) Semaglutide group received saline and Semaglutide (25 nmol/kg ip. once daily for 7 days); (iv) MPTP group received MPTP alone (once daily 20 mg/kg ip. for 7 days); (v) MPTP (once daily 20 mg/kg ip. for 7 days) was immediately followed by NN-2211 treated group (25 nmol/kg ip. once daily for 7 days). (vi) MPTP (20 mg/kg i.p. once daily for 7 days), which was immediately followed by the group treated with semaglutide (25 nmol/kg i.p. once daily for 7 days). Measure behavioral changes, neuronal damage, inflammatory markers, and other biomarkers at the conclusion of drug treatments[2].
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The Cmax of semaglutide was 10.9 nmol/L, with AUC of 3123.4 nmol h/L and a Tmax of 56 h in one clinical trial, achieved within 1-3 days. The absolute bioavailability is 89%. Steady-state concentration of the oral tablet is achieved in 4-5 weeks. Average steady state concentrations of semaglutide are the mean steady state concentrations after dosing at 0.5mg to 1mg range from 16 nmol/L to 30 nmol/L. This drug is mainly cleared by the kidneys, and is found excreted in both the urine and feces. The main elimination route is the urine by corresponding to 53% of an ingested radiolabeled dose, with 18.6% found in the feces. A smaller amount of 3.2% was found to be exhaled. Hepatic impairment does not appear to affect the clearance of this drug and dose adjustments are not required in patients with decreased liver function. The volume of distribution of semaglutide is 8L to 9.4L. It crosses the placenta in rats. The clearance rate of semaglutide is 0.039 L/h according to one clinical study. On the FDA label, semaglutide clearance is reported to be about 0.05 L/h in patients with type 2 diabetes mellitus. Metabolism / Metabolites Semaglutide is cleaved at the peptide backbone, followed by β‐oxidation of the fatty acid chain. Naturally occurring GLP‐1 is quickly metabolized by dipeptidyl peptidase‐4 (DPP‐4) and other enzymes, which is ubiquitous in human tissues. Chemical structure modifications render semaglutide less susceptible to enzymatic degradation by gastrointestinal DPP‐4 enzymes. It is slowly and extensively metabolized, with about 83% of the administered dose measured in the plasma as unchanged drug. Neural endopeptidase (NEP) is another enzyme that metabolizes this drug. DPP-4 inactivates semaglutide, truncating the N-terminal segment while NEP hydrolyzes peptide bondsSix different metabolites of semaglutide have been identified in human plasma. The major metabolite, named P3, accounts for about 7.7% of an ingested dose. Biological Half-Life One of the major properties of semaglutide is its long half-life of 168 h. The long half-life is attributed to its albumin binding. This lowers the renal clearance and protects semaglutide from metabolic breakdown. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large clinical trials, serum enzyme elevations were no more common with semaglutide therapy than with placebo or comparator agents, and no instances of clinically apparent liver injury were reported. Indeed, treatment with semaglutide and other GLP-1 analogues is often associated with improvements in serum aminotransferase levels (and hepatic steatosis) making them possible treatments for nonalcoholic fatty liver. Since licensure, there have been no published case reports of hepatotoxicity due to semaglutide and the product label does not list liver injury as an adverse event. Thus, liver injury due to semaglutide must be rare, if it occurs at all. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of semaglutide during breastfeeding. Because semaglutide is a peptide molecule with a molecular weight of 4113 Da and is over 99% protein bound, the amount in milk is likely to be very low. Furthermore, semaglutide is only 0.4% to 1% orally absorbed, so it is unlikely to adversely affect the breastfed infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. ◈ What is semaglutide? Semaglutide is a medication that has been used to improve blood sugar control in adults with type 2 diabetes. It is available as an injection (given by shot) or by tablet (taken by mouth). The injectable form is sold under the brand name Ozempic®. The tablet form is sold under the brand name Rybelsus®.Semaglutide can also be used as an injection to treat obesity. A brand name for semaglutide used for weight management is Wegovy®. Weight loss is not recommended during pregnancy. Talk with your healthcare providers about using Wegovy® and what treatment is best for you.It is important to talk with your healthcare providers before making any changes to how you take your medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy.Obesity and high/uncontrolled blood sugar can make it harder to get pregnant, and increase the chance of miscarriage, birth defects, or other pregnancy complications. MotherToBaby has fact sheets on diabetes https://mothertobaby.org/fact-sheets/type-1-and-type-2-diabetes/ and obesity https://mothertobaby.org/fact-sheets/obesity-pregnancy/. ◈ I am taking semaglutide, but I would like to stop taking it before getting pregnant. How long does the drug stay in my body? People eliminate medication at different rates. In healthy adults, it can take up to 6 weeks, on average, for most of the semaglutide to be gone from the body. The product labels for Ozempic®, Wegovy®, and Rybelsus® recommend people who are planning a pregnancy to stop this medication 2 months before a pregnancy. ◈ I take semaglutide. Can it make it harder for me to get pregnant? It is not known if semaglutide can make it harder to get pregnant. ◈ Does taking semaglutide increase the chance of miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. Studies have not been done in humans to see if semaglutide increases the chance of miscarriage. Animal studies have reported a higher chance for miscarriage. It is unclear if this finding was due to the medication or from weight loss. As there can be many causes of miscarriage, it is hard to know if the medication, the medical condition, or other factors are the cause of a miscarriage. ◈ Does taking semaglutide increase the chance of birth defects?* Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Research studies have not been done to see if semaglutide increases the chance of birth defects in humans. There has been one report of a person who got pregnant while taking semaglutide. The person stayed on semaglutide for the first 3-4 weeks of pregnancy and gave birth to a child without reported birth defects.In animal studies done by the manufacturer, an increased chance for some birth defects was seen. However, this happened when the amount of semaglutide given was toxic to the mother animal. Also, it is unclear if the reported birth defects were due to the medication or other factors in the study (such as weight loss).Because high/uncontrolled blood sugar can increase the chance of birth defects and other pregnancy complications, it is important to weigh the benefit of using semaglutide against the risks of the untreated condition. Talk with your healthcare provider about the best way to treat your condition in pregnancy. ◈ Does taking semaglutide in pregnancy increase the chance of other pregnancy-related problems? Human studies have not been done to see if semaglutide increases the chance for pregnancy-related problems such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth). Animal studies reported offspring that were smaller than usual when the parent animal was exposed to doses higher than the dose used in humans. It is unclear if this was due to the medication, weight loss, or that the study animals were healthy and did not need to take semaglutide to stay healthy. ◈ Does taking semaglutide in pregnancy affect future behavior or learning for the child? Studies have not been done to see if semaglutide can cause behavior or learning issues for the child. ◈ Breastfeeding while taking semaglutide: There is no available information about semaglutide and human breastmilk. Based on an animal study, semaglutide is expected to get into breastmilk in small amounts. Your healthcare providers can talk with you about using semaglutide and what treatment is best for you. Be sure to talk to your healthcare provider about all your breastfeeding questions.The product label for Rybelsus® recommends that people who are breastfeeding not use the tablet form of the medication if they are breastfeeding an infant. This is because there is a theoretical concern that using the tablet form of this medication could lead to higher levels in a nursing infant. However, the benefit of using semaglutide may outweigh possible risks. Your healthcare provider can talk with you about using semaglutide in these other forms (tablet versus injectable) and what treatment is best for you. ◈ If a male takes semaglutide, could it affect fertility or increase the chance of birth defects? Studies have not been done in humans to see if semaglutide could affect male fertility (ability to get partner pregnant) or increase the chance of birth defects above the background risk. There were no changes in male fertility reported in one animal study using the dose of semaglutide that would be used in humans. In general, exposures that fathers or sperm donors have are unlikely to increase risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. Protein Binding Semaglutide binds with high affinity to plasma albumin, promoting high levels of drug stability. It is more than 99% bound to albumin. |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Semaglutide reduces HbA1c, systolic blood pressure, and body weight. After 12 weeks of treatment, semaglutide decreased fasting and postprandial glucose by increasing insulin production and decreasing glucagon secretion (which is normally associated with increases in blood sugar). Semaglutide also lowers fasting triglycerides and VLDL cholesterol, exerting beneficial effects on cardiovascular health. Semaglutide has been shown to cause medullary thyroid cell carcinoma in rodents. While its clinical relevance to humans is unknown, the FDA advises not to administer this drug in those with a personal or family history of medullary thyroid carcinoma. Semaglutide also poses a risk of pancreatitis and dehydration. Patients must be adequately hydrated while on semaglutide and are advised to seek medical attention immediately in cases of abdominal pain radiating to the back. Because this drug delays gastric emptying, it is important to monitor for the efficacy or adverse effects of other drugs that are administered orally. |
| 分子式 |
C187H291N45O59
|
|---|---|
| 分子量 |
4113.5776
|
| 精确质量 |
4111.12
|
| 元素分析 |
C, 54.60; H, 7.13; N, 15.32; O, 22.95
|
| CAS号 |
910463-68-2
|
| 相关CAS号 |
1997361-85-9 (Semaglutide acetate); 910463-68-2 (Semaglutide free base); 2924330-56-1 (sodium)
|
| PubChem CID |
56843331
|
| 序列 |
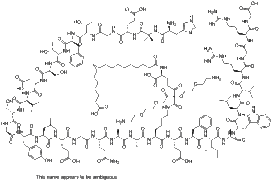
H-His-Aib-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys-Glu-Phe-Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly-OH
|
| 短序列 |
HXEGTFTSDV SSYLEGQAAK EFIAWLVRGR G
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
-5.8
|
| tPSA |
1650Ų
|
| 氢键供体(HBD)数目 |
57
|
| 氢键受体(HBA)数目 |
63
|
| 可旋转键数目(RBC) |
151
|
| 重原子数目 |
291
|
| 分子复杂度/Complexity |
9590
|
| 定义原子立体中心数目 |
30
|
| SMILES |
CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(=N)N)C(=O)NCC(=O)N[C@@H](CCCNC(=N)N)C(=O)NCC(=O)O)NC(=O)[C@H](CC3=CC=CC=C3)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCCCNC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](C(=O)O)NC(=O)CCCCCCCCCCCCCCCCC(=O)O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(=O)N)NC(=O)CNC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC4=CC=C(C=C4)O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC5=CC=CC=C5)NC(=O)[C@H]([C@@H](C)O)NC(=O)CNC(=O)[C@H](CCC(=O)O)NC(=O)C(C)(C)NC(=O)[C@H](CC6=CN=CN6)N
|
| InChi Key |
DLSWIYLPEUIQAV-CCUURXOWSA-N
|
| InChi Code |
InChI=1S/C187H291N45O59/c1-18-105(10)154(180(282)208-108(13)159(261)216-133(86-114-89-200-119-50-40-39-49-117(114)119)170(272)218-129(82-102(4)5)171(273)228-152(103(6)7)178(280)215-121(53-44-72-199-186(192)193)162(264)201-91-141(242)209-120(52-43-71-198-185(190)191)161(263)204-94-151(257)258)230-172(274)131(83-111-45-33-31-34-46-111)219-167(269)126(64-69-149(253)254)214-166(268)122(51-41-42-70-195-144(245)98-290-79-78-289-76-74-197-145(246)99-291-80-77-288-75-73-196-139(240)66-61-127(183(285)286)211-140(241)54-37-29-27-25-23-21-19-20-22-24-26-28-30-38-55-146(247)248)212-158(260)107(12)206-157(259)106(11)207-165(267)125(60-65-138(189)239)210-142(243)92-202-163(265)123(62-67-147(249)250)213-168(270)128(81-101(2)3)217-169(271)130(85-113-56-58-116(238)59-57-113)220-175(277)135(95-233)223-177(279)137(97-235)224-179(281)153(104(8)9)229-174(276)134(88-150(255)256)221-176(278)136(96-234)225-182(284)156(110(15)237)231-173(275)132(84-112-47-35-32-36-48-112)222-181(283)155(109(14)236)227-143(244)93-203-164(266)124(63-68-148(251)252)226-184(287)187(16,17)232-160(262)118(188)87-115-90-194-100-205-115/h31-36,39-40,45-50,56-59,89-90,100-110,118,120-137,152-156,200,233-238H,18-30,37-38,41-44,51-55,60-88,91-99,188H2,1-17H3,(H2,189,239)(H,194,205)(H,195,245)(H,196,240)(H,197,246)(H,201,264)(H,202,265)(H,203,266)(H,204,263)(H,206,259)(H,207,267)(H,208,282)(H,209,242)(H,210,243)(H,211,241)(H,212,260)(H,213,270)(H,214,268)(H,215,280)(H,216,261)(H,217,271)(H,218,272)(H,219,269)(H,220,277)(H,221,278)(H,222,283)(H,223,279)(H,224,281)(H,225,284)(H,226,287)(H,227,244)(H,228,273)(H,229,276)(H,230,274)(H,231,275)(H,232,262)(H,247,248)(H,249,250)(H,251,252)(H,253,254)(H,255,256)(H,257,258)(H,285,286)(H4,190,191,198)(H4,192,193,199)/t105-,106-,107-,108-,109+,110+,118-,120-,121-,122-,123-,124-,125-,126-,127+,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,152-,153-,154-,155-,156-/m0/s1
|
| 化学名 |
18-[[(1R)-4-[2-[2-[2-[2-[2-[2-[[(5S)-5-[[(2S)-2-[[(2S)-2-[[(2S)-5-amino-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-2-[[(2S,3R)-2-[[2-[[(2S)-2-[[2-[[(2S)-2-amino-3-(1H-imidazol-5-yl)propanoyl]amino]-2-methylpropanoyl]amino]-4-carboxybutanoyl]amino]acetyl]amino]-3-hydroxybutanoyl]amino]-3-phenylpropanoyl]amino]-3-hydroxybutanoyl]amino]-3-hydroxypropanoyl]amino]-3-carboxypropanoyl]amino]-3-methylbutanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-methylpentanoyl]amino]-4-carboxybutanoyl]amino]acetyl]amino]-5-oxopentanoyl]amino]propanoyl]amino]propanoyl]amino]-6-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-5-carbamimidamido-1-[[2-[[(2S)-5-carbamimidamido-1-(carboxymethylamino)-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-6-oxohexyl]amino]-2-oxoethoxy]ethoxy]ethylamino]-2-oxoethoxy]ethoxy]ethylamino]-1-carboxy-4-oxobutyl]amino]-18-oxooctadecanoic acid
|
| 别名 |
NN 9535; NN9535; NN-9535; Ozempic; NNC 0113-0217; NNC-0113-0217; NNC0113-0217; Semaglutide; Ozempic; Rybelsus; NN9535; UNII-53AXN4NNHX; Wegovy; NN 9535;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O: ~50 mg/mL (~12.2 mM)
DMSO: ~5 mg/mL (~1.2 mM) |
|---|---|
| 溶解度 (体内实验) |
如何溶解多肽,详情请参考右上角《产品说明书》第3页:“多肽溶解指南”。
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。 注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.2431 mL | 1.2155 mL | 2.4310 mL | |
| 5 mM | 0.0486 mL | 0.2431 mL | 0.4862 mL | |
| 10 mM | 0.0243 mL | 0.1215 mL | 0.2431 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Research Study on How Well Semaglutide Helps Children and Teenagers With Excess Body Weight Lose Weight
CTID: NCT05726227
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-11-19
|
|
|