| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
MCHR1/melanin-concentrating hormonereceptor1 (Ki= 2.2 nM; Kd=530 pM)
|
|---|---|
| 体外研究 (In Vitro) |
本研究描述了放射自显影分析的优化,该分析提供了一种方法来测量黑色素浓缩激素受体1 (MCH(1))拮抗剂在天然组织中的体外效力及其体外受体占用率。初步定位研究表明,MCH(1)受体放射配体[(125)I]-S36057与大鼠尾状壳核结合,特异性结合率始终为60%。在体外实验中,MCH(1)受体拮抗剂GW3430、 nap -94847和4'-{[1-(环丙基甲基)哌啶-4-酰基][5-氟-6-(三氟甲基)- 1h -苯并咪唑-2-基]甲基}联苯-3-碳腈(简称化合物A)对[(125)I]- s36057的特异性结合表现出浓度依赖性的抑制作用,亲和程度顺序为 nap -94847>Compound A>GW3430。[4]
|
| 体内研究 (In Vivo) |
SNAP 94847(口服管饲;20 mg/kg;14 天)显示对急性喹吡罗的过度运动反应[治疗:F(2,19)=11.31,治疗×时间:F(34,323) = 4.061],SNAP 94847 的效果在整个观察期间,与未治疗的动物相比,喹吡罗诱导的活性显着[2]。与未处理的动物相比,饮用水中的 SNAP 94847(口服;20 mg/kg;21 天)显着增加了活动能力 [处理:F(3,28) = 8.971;治疗×时间:F(51,476)=11.50]。结果表明,SNAP 94847 治疗组在 40 分钟后明显增加了运动量,并且这种效果在 180 分钟时仍然显着 [2]。 SNAP 94847(口服;10 mg/kg)具有良好的生物利用度(59%),血浆和血液清除率分别较低,分别为 4.2 L/hr/kg 和 3.3 L/hr/kg,半衰期如 PK 所示研究显示,大鼠的作用持续时间为5.2小时[3]。
黑色素浓缩激素(Melanin-concentrating hormone, MCH)是一种下丘脑神经肽,在调节食物摄入和情绪中起作用。在啮齿动物中,MCH的作用是通过MCHR1受体介导的。本研究的目的是研究急性(1小时)和慢性(28天)p.o.给药一种新型MCHR1拮抗剂N-[3-(1-{[4-(3,4-二氟苯氧基)-苯基]甲基}(4-哌啶基)-4-甲基苯基]-2-甲基丙酰胺(SNAP-94847)对三种预测抗抑郁/抗焦虑样活性的小鼠模型的影响:129S6/SvEvTac小鼠的新颖抑制喂养(NSF)和BALB/cJ小鼠的光/暗范式(L/D)和强迫游泳试验(FST)。急性和慢性治疗SNAP-94847时,观察到L/D盒光室的时间显著增加。在急性和慢性治疗后,NSF测试中发现了抗焦虑/抗抑郁样作用,而在FST中没有观察到任何作用。由于在NSF试验中,齿状回的神经发生已被证明是抗抑郁药作用的必要条件,因此我们研究了SNAP-94847的作用是否需要神经发生。我们发现,用SNAP-94847进行慢性治疗可以刺激齿状回中祖细胞的增殖。然而,在x射线照射抑制神经发生的小鼠中,SNAP 94847在NSF试验中的功效没有改变。这些结果表明,SNAP-94847在急性和慢性给药后都具有独特的抗焦虑样特征,其作用机制与选择性5 -羟色胺再摄取抑制剂和三环抗抑郁药不同。[1] 全身注射SNAP 94847可降低食物增强的操作反应和mch诱导的寻找食物的恢复。SNAP-94847对颗粒启动、cue或育因宾诱导的恢复无影响。 结论:结果表明,MCH1受体参与食物强化的操作性反应,但不参与急性暴露于高脂肪食物、食物线索或育亨宾诱导的应激样状态诱导的恢复。这些结果表明,不同的机制介导食物增强的操作性反应和食物寻找的恢复。[2] |
| 酶活实验 |
Competition binding studies [4]
在尾状壳核水平的冠状切片被用于竞争结合研究。简单地说,在预孵育之后,将切片用50 pM [125I]-S36057孵育,在0.01 nM-10 μM范围内存在MCH1受体拮抗剂(GW3430, snap94847 或化合物a);如前所述,全部溶解并稀释在100% DMSO中,并添加到实验缓冲液中,使DMSO的最终浓度恒定在1%。非特异性结合用1 μM MCH定义。 |
| 动物实验 |
Animal/Disease Models: Rat[2]
Doses: 20 mg/kg Route of Administration: Oral; 20 mg/kg; 14 days Experimental Results: demonstrated excessive locomotor response to acute quinpirole. Animal/Disease Models: Rat (PK study) [3] Doses: 10 mg/kg Route of Administration: po (oral gavage); 10 mg/kg Experimental Results: It demonstrated good physical and chemical properties in rats. SNAP-94847 (3, 10, 15, and 30 mg/kg, intraperitoneal (i.p.)) was dissolved in 20% 2-hydroxypropyl-β-cyclodextrin (encapsin) and yohimbine (2 mg/kg, i.p.) was dissolved in sterile water. [2] Experiment 1: Effect of SNAP-94847 on food-reinforced operant responding [2] We initially studied the effect of systemic injections of SNAP-94847 on ongoing food-reinforced responding. For 14 days, the rats (n=12) were given one 3-h training session as described above. We then assessed the effect of SNAP-94847 on lever presses for the pellets in four 3-h tests that were conducted every 48 h. We used a within-subjects experimental design with the factors of SNAP-94847 dose (vehicle, 3, 10, and 30 mg/kg) and session hour (hours 1, 2, and 3). Each rat was injected with vehicle or one of the doses of SNAP 94847, in a counterbalanced order. SNAP 94847 or its vehicle was injected 60 min prior to the test sessions because previous studies have demonstrated that SNAP-94847 doses of up to 30 mg/kg achieve significant brain penetration by 60 min (DGS unpublished data and Chen et al. 2007). Experiment 2: Effect of SNAP-94847 on MCH-induced reinstatement of food seeking [2] To determine the effect of SNAP-94847 on MCH-induced reinstatement of food seeking, we initially assessed the effect of MCH on this reinstatement. Following our experiment on the effect of systemic injections of SNAP 94847 on food-reinforced responding, we implanted the rats with a guide cannula into the lateral ventricle. After a postoperative recovery period of 5 days, the rats were retrained to lever press for the high-fat food pellets for 3 days and the lever pressing response was extinguished in 13 daily extinction sessions. The rats (n=12) were injected with vehicle or MCH (2.5, 5, 10, and 20μg, i.c.v.) in five test sessions, every 48 h, in an ascending order of MCH dose, with extinction sessions on the intervening days. We used a within-subjects experimental design with the factors of MCH dose (vehicle, 2.5, 5, 10, and 20μg) and session hour. In a different group of rats (n=10), we examined the effect of SNAP-94847 (30 mg/kg, i.p.) on MCH-induced (20μg) reinstatement. We used a within-subjects experimental design with the within-subjects factors of pretreatment condition (0 or 30 mg/kg SNAP 94847), MCH dose (0, 20μg), and session hour. On test days that were separated by 24–72 h, each rat was injected systemically with SNAP-94847 (30 mg/kg) or its vehicle 60 min before the test sessions and then injected with MCH or its vehicle 8–12 min before the sessions; the injections of MCH and its vehicle and SNAP 94847 and its vehicle were counterbalanced. Experiment 3: Effect of SNAP SNAP-94847 on yohimbine-, pellet-priming-, and cue-induced reinstatement of food seeking [2] Pellet-priming-induced reinstatement We tested the effect of SNAP-94847 on pellet-priming-induced reinstatement in four 3-h test sessions with two sessions run consecutively and one extinction day between the two sets of tests. During the test sessions, three food pellets were administered noncontingently within the first minute of the session (i.e., one pellet delivered every 20 s). We used a mixed experimental design that included the between-subject factor of SNAP dose (15 or 30 mg/kg, n=10 in each group) and the within-subjects factors of pretreatment condition (0 and SNAP-94847 [15 or 30 mg/kg]), priming condition (pellet, no pellet), and session hour. On test days that were separated by 24–72 h, each rat was injected systemically with the SNAP-94847 vehicle or one of the SNAP-94847 doses (15 or 30 mg/kg) 60 min before the test sessions and then exposed to the priming condition (three pellets or no pellets); the injections of SNAP 94847 and its vehicle and the priming conditions were counterbalanced. Cue-induced reinstatement [2] As mentioned above, during the training phase, each pellet delivery was paired with a tone–light cue (cue); this cue was not presented during the extinction phase after lever pressing. During the tests for reinstatement, lever responding led to contingent presentations of the cue under the fixed-ratio 1 20-s timeout reinforcement schedule. We tested the effect of SNAP-94847 on cue-induced reinstatement in a total of four test sessions with two sessions run consecutively and five extinction days between test sets. We conducted five extinction days between sets of tests in accordance with previous experiments demonstrating that this procedure minimizes habituation to the presentation of conditioned cues (unpublished data and Bossert et al. 2006; Ghitza et al. 2007). We used a mixed design with between-subject factor of SNAP dose (15 or 30 mg/kg, n=8 in the 15 mg/kg group and n=10 in the 30 mg/kg group) and the within-subjects factors of pretreatment condition (0 and SNAP-94847 [15 or 30 mg/kg]), cue (cue, no cue), and session hour. Yohimbine-induced reinstatement [2] We tested the effect of SNAP-94847 on yohimbine-induced reinstatement in four test sessions with two sessions run consecutively and one extinction day between sets of tests. We used a mixed experimental design that included the between-subject factor of SNAP dose (15 or 30 mg/kg, n=12 in the 15 mg/kg group and n=19 in the 30 mg/kg group) and the within-subjects factors of pretreatment condition (0 and SNAP-94847 [15 or 30 mg/kg]), yohimbine dose (0 or 2 mg/kg), and session hour. Ten rats each in the 15- and 30-mg/kg SNAP-94847 dose were rats previously tested for the effect of SNAP 94847 on pellet-priming-induced reinstatement. These rats were given 2 days of extinction prior to tests for yohimbine-induced reinstatement. On the test days that were separated by 24–72 h, each rat was injected systemically with the SNAP 94847 vehicle or one of the SNAP 94847 doses (15 or 30 mg/kg) 60 min before the test sessions and then injected with yohimbine 15 min later (i.e., 45 min prior to the test session); the injections of SNAP 94847 or its vehicle and yohimbine or its vehicle were counterbalanced. |
| 参考文献 |
|
| 其他信息 |
Rationale and objectives: The melanin-concentrating hormone 1 (MCH1) receptors play an important role in home-cage food consumption in rodents, but their role in operant high-fat food-reinforced responding or reinstatement of food seeking in animal models is unknown. Here, we used the MCH1 receptor antagonist SNAP 94847 to explore these questions.
Materials and methods: In experiment 1, we trained food-restricted rats (16 g/day of nutritionally balanced rodent diet) to lever press for high-fat (35%) pellets (3-h/day, every other day) for 14 sessions. We then tested the effect of SNAP 94847 (3-30 mg/kg, intraperitoneal (i.p.)) on food-reinforced operant responding. In experiments 2 and 3, we trained rats to lever press for the food pellets (9 to 14 3-h sessions) and subsequently extinguished the food-reinforced lever responding by removing the food (10 to 17 sessions). We then tested the effect of SNAP 94847 on reinstatement of food seeking induced by MCH (20 microg, intracerebroventricular), noncontingent delivery of three pellets during the first minute of the test session (pellet-priming), contingent tone-light cues previously associated with pellet delivery (cue), or the pharmacological stressor yohimbine (2 mg/kg, i.p.). [2]
|
| 分子式 |
C29H33CLF2N2O2
|
|---|---|
| 分子量 |
478.58
|
| 精确质量 |
514.22
|
| 元素分析 |
C, 72.78; H, 6.74; F, 7.94; N, 5.85; O, 6.69
|
| CAS号 |
487051-12-7
|
| 相关CAS号 |
SNAP 94847 hydrochloride;1781934-47-1
|
| PubChem CID |
16756754
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
8.429
|
| tPSA |
45.06
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
661
|
| 定义原子立体中心数目 |
0
|
| SMILES |
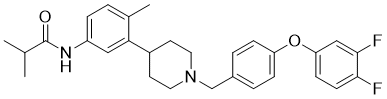
CC1=C(C=C(C=C1)NC(=O)C(C)C)C2CCN(CC2)CC3=CC=C(C=C3)OC4=CC(=C(C=C4)F)F
|
| InChi Key |
VMLZFUVIKCGATC-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C29H32F2N2O2/c1-19(2)29(34)32-23-7-4-20(3)26(16-23)22-12-14-33(15-13-22)18-21-5-8-24(9-6-21)35-25-10-11-27(30)28(31)17-25/h4-11,16-17,19,22H,12-15,18H2,1-3H3,(H,32,34)
|
| 化学名 |
N-[3-[1-[[4-(3,4-difluorophenoxy)phenyl]methyl]piperidin-4-yl]-4-methylphenyl]-2-methylpropanamide
|
| 别名 |
SNAP-94847; SNAP94847; SNAP-94847; 487051-12-7; SNAP 94847; N-(3-{1-[4-(3,4-DIFLUOROPHENOXY)BENZYL]-4-PIPERIDINYL}-4-METHYLPHENYL)-2-METHYLPROPANAMIDE; CHEMBL242004; N-(3-(1-(4-(3,4-difluorophenoxy)benzyl)piperidin-4-yl)-4-methylphenyl)isobutyramide; N-[3-[1-[[4-(3,4-difluorophenoxy)phenyl]methyl]piperidin-4-yl]-4-methylphenyl]-2-methylpropanamide; SNAP 94847
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0895 mL | 10.4476 mL | 20.8951 mL | |
| 5 mM | 0.4179 mL | 2.0895 mL | 4.1790 mL | |
| 10 mM | 0.2090 mL | 1.0448 mL | 2.0895 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。