| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| Other Sizes |

| 靶点 |
PDE5 (IC50 = 90 nM)
|
|---|---|
| 体外研究 (In Vitro) |
PDE5 被顺式他达拉非(化合物 12b)抑制,IC50 为 0.09 μM [1]。
|
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Simultaneous administration of an antacid (magnesium hydroxide/aluminum hydroxide) and tadalafil reduced the apparent rate of absorption of tadalafil without altering the exposure (AUC) to tadalafil. A significant interaction between tadalafil and nitroglycerin was observed to last up to 48 hours; at least 48 hours should elapse after the last dose of tadalafil before nitrate administration is considered. Administration of tadalafil to patients who are using any form of organic nitrates, either regularly and/or intermittently, is contraindicated; in clinical pharmacology studies tadalafil was shown to potentiate the hypotensive effects of nitrates; this is thought to result from the combined effects of nitrates and tadalafil on the nitric oxide/cGMP pathway. The safety and efficacy of combinations of tadalafil and other erectile dysfunctions have not been studied; use of combinations is not recommended. For more Interactions (Complete) data for TADALAFIL (15 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Modification of the hydantoin ring in the previously described lead compound 2a has led to the discovery of compound 12a, tadalafil, a highly potent and highly selective PDE5 inhibitor. The replacement of the hydantoin in compound 2a by a piperazinedione ring led to compound cis-11a which showed similar PDE5 inhibitory potency. Introduction of a 3,4-methylenedioxy substitution on the phenyl ring in position 6 led to a potent PDE5 inhibitor cis-11c with increased cellular potency. Optimization of the chain on the piperazinedione ring led to the identification of the racemic cis-N-methyl derivative 11i. High diastereospecificity for PDE5 inhibition was observed in the piperazinedione series with the cis-(6R,12aR) enantiomer displaying the highest PDE5 inhibitory activity. The piperazinedione 12a, tadalafil (GF196960), has been identified as a highly potent PDE5 inhibitor (IC(50) = 5 nM) with high selectivity for PDE5 vs PDE1-4 and PDE6. Compound 12a displays 85-fold greater selectivity vs PDE6 than sildenafil 1. 12a showed profound and long-lasting blood pressure lowering activity (30 mmHg/>7 h) in the spontaneously hypertensive rat model after oral administration (5 mg/kg).
Therapeutic Uses Tadalafil is indicated for the treatment of erectile dysfunction. /Included in US product labeling/ Drug Warnings The Food and Drug Administration ... approved updated labeling for Cialis, Levitra and Viagra to reflect a small number of post-marketing reports of sudden vision loss, attributed to NAION (non arteritic ischemic optic neuropathy), a condition where blood flow is blocked to the optic nerve. FDA advises patients to stop taking these medicines, and call a doctor or healthcare provider right away if they experience sudden or decreased vision loss in one or both eyes. Further, patients taking or considering taking these products should inform their health care professionals if they have ever had severe loss of vision, which might reflect a prior episode of NAION. Such patients are at an increased risk of developing NAION again. Cardiovascular status of patients should be considered since there is a degree of risk associated with sexual activity; treatments for erectile dysfunction, including tadalafil, should not be used in men for whom sexual activity is inadvisable as a result of their underlying cardiac status. The following groups of patients with cardiovascular disease were not included in clinical safety and efficacy trials for Cialis, and, therefore, the use of Cialis is not recommended in these groups until further information is available: patients with a myocardial infarction within the last 90 days, patients with unstable angina or angina occurring during sexual intercourse, patients with New York Heart Association Class 2 or greater heart failure in the last 6 months, patients with uncontrolled arrhythmias, hypotension (<90/50 mm Hg), or uncontrolled hypertension (>170/100 mm Hg), and patients with a stroke within the last 6 months. In addition, patients with known hereditary degenerative retinal disorders, including retinitis pigmentosa, were not included in the clinical trials, and use in these patients is not recommended. The effect of a 100 mg single dose of tadalafil on the QT interval was evaluated at the time of peak tadalafil concentration in a randomized, double-blinded, placebo, and active (intravenous ibutilide)-controlled crossover study in 90 healthy males aged 18 to 53 years. The mean change in QTc (Fridericia QT correction) for tadalafil, relative to placebo, was 3.5 milliseconds (two-sided 90% CI=1.9, 5.1). The mean change in QTc (Individual QT correction) for tadalafil, relative to placebo, was 2.8 milliseconds (two-sided 90% CI=1.2, 4.4). A 100 mg dose of tadalafil (5 times the highest recommended dose) was chosen because this dose yields exposures covering those observed upon coadministration of tadalafil with potent CYP3A4 inhibitors or those observed in renal impairment. In this study, the mean increase in heart rate associated with /this/ dose of tadalafil compared to placebo was 3.1 beats per minute. For more Drug Warnings (Complete) data for TADALAFIL (18 total), please visit the HSDB record page. Pharmacodynamics Tadalafil exerts a therapeutic effect in ED by increasing sexual stimulation-dependant smooth muscle relaxation in the penis, allowing the corpus cavernosum to fill with blood to produce an erection. Smooth muscle relaxation in the pulmonary vasculature helps to produce vasodilation in PAH which reduces blood pressure in the pulmonary arteries. In BPH, tadalafil may contribute to decreased smooth muscle cell proliferation which may reduce the size of the prostate and relieve the anatomical obstruction which produces urinary symptoms of BPH. The decreased affinity of tadalafil for PDE6 compared to other PDE5 inhibitors may explain the reduced incidence of visual side effects. |
| 分子式 |
C22H19N3O4
|
|---|---|
| 分子量 |
389.40396
|
| 精确质量 |
389.138
|
| 元素分析 |
C, 67.86; H, 4.92; N, 10.79; O, 16.43
|
| CAS号 |
171596-27-3
|
| 相关CAS号 |
Nortadalafil;171596-36-4;Tadalafil;171596-29-5;ent-Tadalafil;629652-72-8;cis-ent-Tadalafil;171596-28-4;cis-Tadalafil-d3;1329799-70-3;cis-ent-Tadalafil-d3
|
| PubChem CID |
9821704
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.51±0.1 g/cm3 (20 ºC 760 Torr)
|
| 熔点 |
295-296 ºC
|
| LogP |
2.087
|
| tPSA |
74.87
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
702
|
| 定义原子立体中心数目 |
2
|
| SMILES |
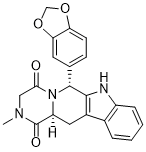
CN1CC(=O)N2C(C1=O)CC3=C(C2C4=CC5=C(C=C4)OCO5)NC6=CC=CC=C36
|
| InChi Key |
WOXKDUGGOYFFRN-IIBYNOLFSA-N
|
| InChi Code |
InChI=1S/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21-/m1/s1
|
| 化学名 |
(6R,12aS)-6-(1,3-Benzodioxol-5-yl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino(1',2'
|
| 别名 |
Tadalafil specified
impurity A, 171596-27-3; Tadalafil, (6R ,12aS)-; cis-Tadalafil; Tadalafil 6R ,12as diastereomer; (-)-Tadalafil 6R ,12as diastereomer; CHEMBL139028; E319TQ0B6R; Tadalafil EP Impurity A; Tadalafil (6R ,12aS)- Tadalafil (6R ,12aS)- Lilly
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5681 mL | 12.8403 mL | 25.6805 mL | |
| 5 mM | 0.5136 mL | 2.5681 mL | 5.1361 mL | |
| 10 mM | 0.2568 mL | 1.2840 mL | 2.5681 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Use of a Phosphodiesterase Type 5 Inhibitor to Improve Anabolic Resistance in Older Adults
CTID: NCT05458232
Phase: Status: Withdrawn
Date: 2024-11-04