| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
|
|
|---|---|---|
| 体外研究 (In Vitro) |
在3T3-L1前脂肪细胞分化的早期和晚期,他氟前列素酸(10、100 nM)有效抑制脂肪生成[2]。在野生型小鼠的原代脂肪细胞中,他氟前列素酸 (100 nM) 会减少脂肪生成,但在 FP 敲除小鼠的原代脂肪细胞中则不然[2]。当暴露于他氟前列素酸 (10-4 M) 六小时时,人脐血管内皮细胞 (HUVEC) 会被刺激增殖和迁移 [4]。刺激 HUVEC 管形成(-4 M,4–18 小时)[4]。
|
|
| 体内研究 (In Vivo) |
在滴注后4-8小时,Taf/T-FDC的眼压降低效果几乎与Lat/T-FDC相当。Taf/T-FDC和Lat/T-FDC在滴注后8小时观察到峰值眼压降低,两者之间没有差异。滴注后24-30小时观察到它们之间的差异,滴注后24-30h,Taf/T-FDC的降眼压效果明显优于Lat/T-FDC。Taf/T-FDC的降眼压作用在滴注后持续到30小时,而Lat/T-FDC的降眼压效果在滴注28小时后几乎消失。Taf/T-FDC滴注后房水中的噻吗洛尔浓度高于Lat/T-FDC滴注后的浓度(Cmax,3870 ng/mL vs 1330 ng/mL;AUCinf,3970 ng·h/mL vs 1250 ng·h/mL)。分别滴注Taf/T-FDC和Lat/T-FDC后,房水中塔氟前列素酸/AFP-172和拉坦前列素酸的浓度与分别滴注塔氟前列醇和拉坦前列腺素单一制剂后的浓度相似。在所有评估的时间点,Taf/T-FDC对人角膜上皮细胞的细胞毒性作用在未稀释和10倍稀释的FDC中均显著低于Lat/T-FDC[3]。
|
|
| 酶活实验 |
为了评估新型前列腺素(PG)F(2α)衍生物AFP-168(他氟前列素)的药理学特征,我们检测了其受体结合亲和力、降低眼压的作用、对房水动力学的影响以及对黑色素生成的刺激作用。通过测量器官浴中的肌肉收缩、血小板聚集的抑制和放射性标记配体的竞争性结合,确定了AFP-168的羧酸塔氟前列酸/AFP-172的受体结合谱。在眼压测量研究中,使用了眼部正常血压和激光诱导的眼部高血压食蟹猴,并使用气相色谱仪测量了眼压。为了研究房水动力学,在眼部血压正常的猴子身上使用了眼压(Goldmann压平眼压计)、荧光光度计、两级恒压灌注和同位素稀释和累积技术。测定培养的B16-F0黑色素瘤细胞的培养基和细胞体中的黑色素含量。Tafluprost酸/AFP-172(Ki:0.4 nm)对FP受体的亲和力是拉坦前列素羧酸PhXA85(Ki:4.7 nm)的12倍。单次应用0.0025%的AFP-168可显著降低眼压正常和高血压猴子的眼压(分别为3.1和11.8 mmHg,p<0.01),0.005%的拉坦前列素可显著降低眼内压(分别为2.1 mmHg,p<0.01和9.5 mmHg,p=0.059)。在血压正常的猴子中,每天滴注0.001、0.0025或0.005%的AFP-168 5天,不仅在几个小时内,而且在用药后24小时的药物低谷时间,眼压都显著降低。0.005%的拉坦前列素也能降低眼压,但在药物低谷时没有。AFP-168主要通过将葡萄膜巩膜流出量增加65%(p<0.05)来降低眼压,并且与其他前列腺素有时一样,还增加了总流出设施(增加33%,p<0.05)。在培养的B16-F0黑色素瘤细胞中,Tafluprost酸/AFP-172(100微M)不会刺激黑色素生成,但PhXA85(100微m)会。这些发现表明,AFP-168对前列腺素FP受体具有很高的亲和力,在眼部正常血压和高血压猴子中都具有比拉坦前列素更强的眼压降低作用,对黑色素瘤细胞中黑色素生成的刺激作用较小[1]。
|
|
| 细胞实验 |
处理3T3-L1前脂肪细胞以促进分化为成熟脂肪细胞。在分化的早期和晚期(第0、2和7天),将1至1000 nM的拉坦前列酸(LAT-A)、曲伏前列酸(TRA-A)、Tafluprost acid(TAF-A)、比马前列素(BIM)、比马前列素酸(BIM-A)、乌诺前列酮(UNO)或前列腺素F2a(PGF2α)应用于细胞。在第10天使用油红O染色检测细胞内脂质。将照片上测量的染色区域与对照培养物中的染色区域进行比较。所有实验均以蒙面方式进行。接下来,使用来自FP受体敲除的原代培养小鼠脂肪细胞和野生型小鼠进行了类似的实验。
结果:当在第0天或第2天添加PG时,LAT-A、TAF-A、BIM-A和PGF2α在10 nM和100 nM的浓度下显著抑制脂肪生成(第0天P<0.01,第2天P<0.05),TRA-A仅在100 nM时抑制脂肪生成。比马前列素和UNO在任何浓度下都不影响脂肪生成。当在第7天添加PG时,100 nM LAT-A、BIM-A或PGF2α显著抑制了脂肪生成(P<0.05)。在小鼠原代脂肪细胞培养中,LAT-A、TAF-A、BIM-A、TRA-A和PGF2α显著抑制了野生型脂肪细胞的脂肪生成(P<0.05),但FP敲除小鼠脂肪细胞中的任何PG化合物都没有抑制脂肪生成。
结论:前列腺素类似物具有通过FP受体刺激抑制脂肪生成的潜力。尽管这些发现应在与眼眶脂肪更密切相关的模型系统中进一步分析,但PG类似物可能通过抑制脂肪生成直接导致眼眶脂肪减少[2]。
在存在或不存在FP受体拮抗剂(10 nM AL-8810)的情况下培养的HUVEC暴露于浓度逐渐升高的10(-7)、10(-6)、10。对于细胞增殖测定,细胞数量由微孔板阅读器的CellTiter96®水性单溶液细胞增殖测定得出。使用FluoroBlok™24孔插入物通过BD Biocoat™血管生成系统评估内皮细胞迁移。BioTek FLx800荧光平板阅读器用于定量测量荧光标记的侵袭性血管内皮细胞。使用Matrigel Matrix 96孔板,通过BD生物涂层血管生成系统评估内皮毛细血管样管的形成。实时定量逆转录聚合酶链式反应(RT-PCR)用于评估血管内皮生长因子(VEGF)、环氧化酶-2(COX-2)和内皮一氧化氮合酶(eNOS)的基因表达。通过免疫荧光染色和Western blot检测COX-2蛋白。学生t检验用于统计分析。[4] |
|
| 动物实验 |
The IOP-lowering effect of Taf/T-FDC and Lat/T-FDC in ocular normotensive monkeys was evaluated at 4 and 8 h after instillation in study A, at 12, 14, 16, and 18 h after instillation in study B, and at 24, 26, 28, and 30 h after instillation in study C. Drug penetration into the eye was evaluated by measuring the concentrations of timolol, Tafluprost acid/AFP-172 (active metabolic form of tafluprost), and latanoprost acid (active metabolic form of latanoprost) using liquid chromatography coupled with tandem mass spectrometry after single instillation of Taf/T-FDC or Lat/T-FDC to Sprague Dawley rats. Cytotoxicity following 1-30 min exposure of SV40-transformed human corneal epithelial cells to Taf/T-FDC or Lat/T-FDC was analyzed using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assays. Undiluted and 10-fold diluted solutions of each FDC were evaluated[3].
|
|
| 药代性质 (ADME/PK) |
Tafluprost acid concentrations demonstrated similar profiles after instillation of Taf/T-FDC and tafluprost mono-preparation, reaching Cmax at 0.25–0.5 h and declining with a T1/2 of 0.43–0.45 h. Latanoprost acid concentrations, which also demonstrated similar profiles after instillation of Lat/T-FDC and latanoprost mono-preparation, reached Cmax at 0.5 h and declined with a T1/2 of 0.35–0.40 h.[3]
|
|
| 参考文献 |
|
|
| 其他信息 |
COX is a key enzyme in the conversion of arachidonic acid to prostaglandin. There are two isoforms of COX such as COX-1 and COX-2. Whereas COX-1, a house-keeping gene, is expressed in most tissues, COX-2, an inducible intermediate-early gene, is induced in inflammatory cells and its role is related with angiogenesis and carcinogenesis. In the current study, 10−4 M Tafluprost acid/AFP-172 stimulated only the gene expression of COX-2 among the three genes. COX-2 protein induced by Tafluprost acid/AFP-172 was blocked in the FP receptor antagonist-pretreated HUVECs. These results suggest that Tafluprost acid/AFP-172 induce COX-2 via FP receptor in HUVECs like the effect of PGF2α in the endometrial adenocarcima. Although COX-2 is well known to promote angiogenesis, it is still unknown how COX-2 mediated angiogensis is involved in the angiogenic process. Unlike COX-1 inhibitor, COX-2 inhibitor is considered to be a potential therapeutic agent for inhibiting angiogenesis and carcinogenesis. In our study, COX-2 inhibitor abolished the angiogenic effects of Tafluprost acid/AFP-172 by reducing proliferation, migration and tube formation of HUVECs. These results suggest that Tafluprost acid/AFP-172 promote angiogenesis in HUVECs through interaction with COX-2 signal transduction pathways. COX-2 and VEGF appeared to be involved in angiogenesis by dual interdependent gene expression pathways. Although COX-2 is known to modulate angiogenesis by interacting with VEGF system, we could not find a difference of expression of VEGF-A in AFP-172-treated cells compared with control by using RT-PCR (Fig. 5). From these data, the angiogenic effects of Tafluprost acid/AFP-172 did not associated with VEGF-A and eNOS. In ocular angiogenesis, hypoxia stimulates angiogenesis by interacting with VEGF, COX-2 and NOS system. We can deduce that the angiogenic mechanism of Tafluprost acid/AFP-172 does not depend on hypoxia-induced ocular angiogenesis but on COX-2-mediated prostanoid biosynthesis via FP receptor. In real clinical situation, 10−4 M is much higher than therapeutic concentration of Tafluprost acid/AFP-172. Furthermore, the amount of eye drops which penetrates to the vitreous through cornea is suggested to be of the order of 1/104 of the dose that reaches the vitreous. Because 1 drop of tafluprost 0.0015 % contains about 2.5 μg tafluprost, 250 pg can be existed in the vitreous. 0.6 × 10−12 M Tafluprost acid/AFP-172 can be expected to reach the vitreous cavity by a single instillation at clinical dose considering that the molecular weight of tafluprost is 452.5 . Based on our results that a higher concentration of 10−4 M Tafluprost acid/AFP-172 could stimulate the angiogenesis of HUVECs, we speculate that the therapeutic dosage of tafluprost may be unable to stimulate the angiogenesis of glaucoma patients associated with both anterior and posterior pathological angiogenic diseases such as neovascular glaucoma, corneal inflammatory diseases, AMD and diabetic retinopathy. But our results of in vitro experiments cannot be applied to treat patients with especially posterior segment angiogenic diseases such as AMD and diabetic retinopathy in some respects. First, aside from endothelial cells in the eye, ocular angiogenesis is also related to other ocular tissues such as retinal pigment epithelium and choroid. Second, although some investigations have been conducted to study ocular pathophysiology and pathogenesis using HUVECs, the similarity between HUVECs and endothelial cells in the eye is little known. However due to the concentration of 10−4 M Tafluprost acid/AFP-172 is still about ten thousand times higher than the pharmaceutical concentrations of tafluprost considering the therapeutic concentration of 1 drop of tafluprost is 0.6 × 10−8 M, tafluprost does not appear to stimulate anterior segment angiogenic process such as neovascular glaucoma, corneal infections and corneal keratoplasty. Our results are rather dissimilar to the angiogenic effect of latanoprost in a rat corneal model. It may be due to different drug compounds and different experimental models that were used. Tafluprost acid/AFP-172, the active carboxyl acid form of tafluprost, differs from latanoprost because tafluprost has two fluorine atoms instead of a hydroxyl group at the carbon 15 position. It is conceivable that our in vitro results may not be similar to in vivo results obtained in the animal study such as a rat corneal angiogenesis assay which was applied to the study of latanoprost. In summary, we demonstrated that Tafluprost acid/AFP-172 has an angiogenic effect by inducing COX-2 protein on HUVECs. For the clinical use especially for glaucoma patients with ocular neovascular diseases, more experiments including in vivo study are needed to investigate the angiogenic mechanism of tafluprost.
|
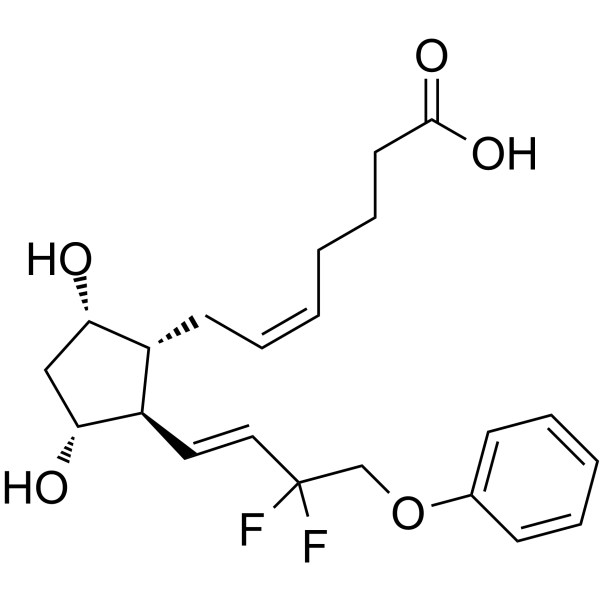
| 分子式 |
C22H28F2O5
|
|---|---|
| 分子量 |
410.46
|
| 精确质量 |
410.19
|
| 元素分析 |
C, 64.38; H, 6.88; F, 9.26; O, 19.49
|
| CAS号 |
209860-88-8
|
| PubChem CID |
9978917
|
| 外观&性状 |
Colorless to light yellow ointment
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
575.9±50.0 °C at 760 mmHg
|
| 闪点 |
302.1±30.1 °C
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
| 折射率 |
1.579
|
| LogP |
2.8
|
| tPSA |
86.99
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
557
|
| 定义原子立体中心数目 |
4
|
| SMILES |
C(=C/C[C@@H]1[C@@H](/C=C/C(COC2=CC=CC=C2)(F)F)[C@@H](C[C@@H]1O)O)/CCCC(=O)O
|
| InChi Key |
KIQXRQVVYTYYAZ-VKVYFNERSA-N
|
| InChi Code |
InChI=1S/C22H28F2O5/c23-22(24,15-29-16-8-4-3-5-9-16)13-12-18-17(19(25)14-20(18)26)10-6-1-2-7-11-21(27)28/h1,3-6,8-9,12-13,17-20,25-26H,2,7,10-11,14-15H2,(H,27,28)/b6-1-,13-12+/t17-,18-,19+,20-/m1/s1
|
| 化学名 |
(Z)-7-[(1R,2R,3R,5S)-2-[(E)-3,3-difluoro-4-phenoxybut-1-enyl]-3,5-dihydroxycyclopentyl]hept-5-enoic acid
|
| 别名 |
UNII-WTV8EPZ396; Tafluprost acid; 209860-88-8; AFP-172; Tafluprost (free acid); WTV8EPZ396; UNII-WTV8EPZ396; 5-Heptenoic acid, 7-[(1R,2R,3R,5S)-2-[(1E)-3,3-difluoro-4-phenoxy-1-buten-1-yl]-3,5-dihydroxycyclopentyl]-, (5Z)-; 5-Heptenoic acid, 7-((1R,2R,3R,5S)-2-((1E)-3,3-difluoro-4-phenoxy-1-buten-1-yl)-3,5-dihydroxycyclopentyl)-, (5Z)-;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~243.64 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4363 mL | 12.1815 mL | 24.3629 mL | |
| 5 mM | 0.4873 mL | 2.4363 mL | 4.8726 mL | |
| 10 mM | 0.2436 mL | 1.2181 mL | 2.4363 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。