| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
VEGFR2 (IC50 = 6.5 nM); VEGFR3 (IC50 = 15 nM); EphB2 (IC50 = 24 nM); VEGFR1 (IC50 = 30 nM); PDGFRα (IC50 = 40 nM)
|
|---|---|
| 体外研究 (In Vitro) |
AV-951是一种新型的尿素和喹啉衍生物。AV-951可阻止内皮细胞增殖和vegf依赖性的有丝分裂原活化蛋白激酶的激活。[1]
KRN951是一种针对vegfr的新型酪氨酸激酶抑制剂,具有抗肿瘤血管生成和抗生长活性。KRN951在体外亚纳摩尔IC50值(IC50 = 0.16 nmol/L)下有效抑制内皮细胞中vegf诱导的VEGFR-2磷酸化。它还能抑制配体诱导的血小板衍生生长因子受体β (pdgfr - β)和c-Kit的磷酸化(IC50分别为1.72和1.63 nmol/L)。KRN951阻断了vegf依赖性而非非依赖性的丝裂原活化蛋白激酶的激活和内皮细胞的增殖。此外,还能抑制vegf介导的人脐静脉内皮细胞的迁移。[1] |
| 体内研究 (In Vivo) |
体内研究表明,特别是口服剂量为1mg /kg的AV-951,还可以降低肿瘤异种移植物的微血管密度并抑制VEGFR2磷酸化水平。在胸腺发育不全的大鼠中,AV-951几乎完全抑制异种肿瘤的生长(TGI>85%)。[1]另一项使用大鼠腹膜播散性肿瘤模型的研究表明,AV-951可以延长荷瘤大鼠MST后的生存期,最长可达53.5天。当应用于各种人类肿瘤异种移植物,如肺癌、乳腺癌、结肠癌、卵巢癌、胰腺癌和前列腺癌时,AV-951表现出抗肿瘤活性。[2]
给胸大鼠po后,KRN951降低了异种肿瘤移植物内的微血管密度,并减弱了肿瘤内皮中VEGFR-2的磷酸化水平。它也显示抗肿瘤活性,对多种人类肿瘤异种移植物,包括肺癌,乳腺癌,结肠癌,卵巢癌,胰腺癌和前列腺癌。此外,动态对比增强磁共振成像(DCE-MRI)分析显示,肿瘤血管高通透性的显著降低与KRN951的抗肿瘤活性密切相关。这些发现表明,KRN951是一种高效的抗血管生成和抗肿瘤药物,DCE-MRI将有助于在临床环境中检测KRN951的早期反应。KRN951目前处于I期临床开发阶段,用于治疗晚期癌症患者。[1] |
| 酶活实验 |
AV-951 针对各种重组受体和非受体酪氨酸激酶(例如 VEGFR1、VEGFR2、VEGFR3、c-Kit、PDGFRβ、Flt-3 和 FGFR1)的 IC50 值通过使用 1 进行四次无细胞激酶测定来确定。 μM ATP。
激酶选择性。[1] 在1 μmol/L ATP条件下进行四次无细胞激酶试验,测定Tivozanib (AV951; KRN-951)的IC50值;Tivozanib (AV951; KRN-951)抗多种重组受体和非受体酪氨酸激酶。重组酶来自ProQinase GmbH。 [1] 以细胞为基础的试验确定Tivozanib (AV951; KRN-951);如前所述,Tivozanib (AV951; KRN-951)抑制受体酪氨酸激酶的配体依赖性磷酸化。简单地说,将细胞在含有0.5%胎牛血清(FBS)的适当基本培养基中饥饿过夜。加入Tivozanib (AV951; KRN-951)或0.1% DMSO后,细胞孵育1小时,然后用同源配体在37℃下刺激。除VEGFR-3(10分钟)、c-Met(10分钟)和c-Kit(15分钟)外,受体磷酸化诱导时间为5分钟。除VEGF-C(一种大鼠重组蛋白)外,实验中使用的所有配体均为人重组蛋白。细胞裂解后,用适当的抗体对受体进行免疫沉淀,并用磷酸酪氨酸进行免疫印迹。印迹的定量和IC50值的计算如前所述进行。 [1] 丝裂原活化蛋白激酶活化。[1] 按照前面的描述对其进行了评估。简单地说,HUVECs在含有0.5% FBS的基本培养基(EBM-2)中饥饿16小时。Tivozanib (AV951; KRN-951)作用1小时,用50 ng/mL VEGF、25 ng/mL碱性成纤维细胞生长因子或20 ng/mL EGF刺激HUVECs。细胞裂解液进行SDS-PAGE,然后用磷酸化的p44/42丝裂原活化蛋白激酶(MAPK)抗体对磷酸化的MAPKs进行免疫印迹。[1] 通透性试验和VEGFR‐2磷酸化检测。[2] 恶性腹水对内皮细胞通透性和VEGFR‐2磷酸化的影响,以及Tivozanib (AV951;KRN-951)对这些影响进行了评价。将第25天从经载体处理的腹膜播散性肿瘤模型中采集的腹水样本进行汇总,并将所得上清用于这些实验。我们检查碘化丙啶摄取作为通透性的措施在体外测定。western blotting检测VEGFR‐2磷酸化水平。为了进行渗透性试验,将HUVEC培养在90%的融合度下,在含有0.5%胎牛血清的基本培养基(EBM‐2)中进行血清饥饿过夜。然后用磷酸盐缓冲盐水、恶性腹水和10 nM浓度的Tivozanib (AV951; KRN-951);培养板中加入Tivozanib (AV951; KRN-951)。单独培养基或50 ng/mL无腹水的VEGF作为内部对照。孵育7 h后,收获细胞,碘化丙啶(1µg/mL)处理,进行FACS分析。通过测定HUVEC对碘化丙啶的吸收来评估其通透性。对于western blotting,除了用腹水刺激时间为10分钟外,采用相同的方法处理HUVEC。细胞裂解后,用抗VEGFR - 2抗体免疫沉淀VEGFR蛋白,然后用抗磷酸酪氨酸抗体免疫印迹,如前所述。[2] |
| 细胞实验 |
使用基于人脐静脉内皮细胞 (HUVEC) 和正常人真皮成纤维细胞的测定来评估 Tivozanib (AV951/KRN-951) 抑制酪氨酸激酶受体配体依赖性磷酸化的能力。在含有 0.5% 胎牛血清 (FBS) 的适当基础培养基中,细胞在第二天处于饥饿状态。将细胞与 Tivozanib (AV951/KRN-951) 或 0.1% DMSO 一起孵育一小时后,在 37 °C 下用同源配体刺激。除了 VEGFR3、c-Met 和 c-Kit 分别诱导 10 分钟和 15 分钟外,受体磷酸化持续 5 分钟。 VEGF-C 是一种大鼠重组蛋白,是检测中使用的唯一非人重组蛋白的配体。细胞裂解后,用适当的抗体进行免疫沉淀后,对受体进行磷酸酪氨酸免疫印迹。印迹定量和IC50值计算均已完成。
内皮细胞增殖。[1] 将HUVECs接种于含5% FBS的M-199中,以4000个细胞/200 μL/孔的密度接种于胶原包被的96孔板中。24小时后,加入Tivozanib (AV951/KRN-951),然后加入20 ng/mL VEGF或10 ng/mL bFGF,培养72小时。加入胸腺嘧啶(1 μCi/mL) [3H],继续培养12小时。然后收集细胞,用液体闪烁计数器测量其放射性。[1] 采用96孔微室板评估HUVEC迁移。细胞在含有0.1%牛血清白蛋白(BSA)的EBM-2中饥饿5小时。然后,收集细胞,在含有0.1% BSA的EBM-2中重悬,并置于上腔。将含有10 ng/mL VEGF、0.1% FBS和0.1% BSA的培养基置于底室,开始细胞迁移。当有指示时,在上、下腔均加入Tivozanib (AV951/KRN-951)。孵育22 h后,用4 μg/mL钙黄素AM染色HBSS细胞。在激发/发射波长为485/530 nm的荧光板阅读器中,通过腔室底部直接测量通过荧光阻断膜孔迁移的细胞的荧光。[1] 细胞毒性检测。[1] 这些试验按前面所述进行。简单地说,将细胞接种于96孔板中,并在含有10%胎牛血清的培养基中培养。 在开始培养后约24小时加入Tivozanib (AV951/KRN-951),细胞孵育72小时。采用WST-1试剂检测细胞活力[1]。 |
| 动物实验 |
Mice: The athymic rats receive a subcutaneous injection of cancer cells in their right flank. Tumors up to 1,500 mm3 are surgically removed, and smaller pieces (20–30 mg) are s.c. implanted into the right flank of rats exposed to radiation. Beginning on day zero of randomization, oral administration of KRN951 (0.2 or 1 mg/kg) or the vehicle is administered. Using Vernier calipers, tumor volume is measured and computed twice a week.
Tumor xenograft models. Athymic rats (RH-rnu/rnu) were used. Twenty-four hours after whole body irradiation with a γ-source (7 Gy, Co60), cancer cells were s.c. inoculated into the right flank of the rats. Once established, tumors of ∼1,500 mm3 were surgically excised and smaller tumor fragments (20-30 mg) were s.c. implanted in the right flank of irradiated rats. Oral administration of Tivozanib (AV951/KRN-951) (0.2 or 1 mg/kg) or vehicle was initiated at the day of randomization (day 0). Tumor volume was measured twice weekly with Vernier calipers, and calculated as (length × width2) × 0.5. Relative tumor volume (RTV) was calculated by the formula: RTV at day x = tumor volume at day x / tumor volume at day 0. Percentage tumor growth inhibition (TGI%) was calculated as described previously. Statistical analysis of RTV was done using the unpaired t test. DCE-MRI. Athymic rats (RH-rnu/rnu) were s.c. implanted with fresh Calu-6 tumor fragments. Rats were randomized when the tumors reached a volume of 274 to 287 mm3 (day −1). Once-daily p.o. administration of Tivozanib (AV951/KRN-951) or vehicle was initiated the day after randomization (day 0) and continued for 2 weeks (days 0-13). MRI experiments were carried out at 1.5 T on a whole body magnet equipped with a flexible receiver coil (circularly polarized). DCE-MRI acquisitions were done on day −1 (before the start of treatment), day 2, day 13, and day 21. On days 2 and 13, the rats were imaged 4 hours after p.o. administration of Tivozanib (AV951/KRN-951). The rat tail vein was cannulated for contrast agent injection before placing the animals in the magnet. During the experiment, rats were anesthetized by an i.m. injection of a mixture of ketamine and xylazine (2/1, v/v, 70 and 15 mg/kg, respectively). The anesthetized rats were placed in a cradle supine position inside the resonator. The exact position of the rats was assessed by a scout imaging sequence. [1] Measurement of tumor vessel diameter. During the MRI study, an additional three groups of Calu-6 tumor-bearing rats (RH-rnu/rnu, three rats per group) were used for measurement of tumor vessel diameter using the fluorescent dye H33342 (24). Rats were treated with Tivozanib (AV951/KRN-951) (0.2 or 1 mg/kg) or vehicle for 14 days (from day 0 to day 13) and were sacrificed 1 minute after the i.v. injection of H33342 (20 mg/kg) at day 13. The tumors were removed and 10 μm cryosections prepared from five levels of each tumor separated by at least 200 μm. Tumor sections were studied under UV illumination using a Nikon epifluorescence microscope to identify blood vessels with a surrounding halo of fluorescent H33342-labeled cells. The lumen enclosed by the halos was measured as the vessel diameter using Win ROOF software. Statistical analysis was done using the Mann-Whitney test.[1] Histologic analysis of smooth muscle actin–positive pericyte coverage of tumor vessels. Calu-6 tumor xenografts were established in athymic rats by s.c. implantation of cells. Rats were randomized when tumor volumes reached an average of 273 to 275 mm3 and then treated p.o. with Tivozanib (AV951/KRN-951) or vehicle for 2 weeks. Immunofluorescence staining of pericytes in tumors was done with Cy3-conjugated monoclonal anti-α-smooth muscle actin antibody following the staining of endothelial cells with anti-CD31 antibody. Tissue images were captured digitally at ×100 magnification with LSM 510 systems. Six fields per section (0.8489 mm2 each) were randomly analyzed, excluding peripheral surrounding connective tissues and central necrotic tissues. The number of CD31-positive objects and those surrounded by the region positive for α-smooth muscle actin were quantified using Win ROOF software after blind-coding the histology slides to avoid operator bias.[1] Pharmacokinetic analysis of Tivozanib (AV951/KRN-951). Athymic rats (F344/N JcL-rnu, four females per group) received Tivozanib (AV951/KRN-951) p.o. and blood samples were collected from their tail vein at predetermined intervals up to 72 hours postdose. An appropriate amount of internal standard material, KRN633, was added to each serum sample. Serum samples were deproteinated with acetonitrile and supernatants were analyzed by high-performance liquid chromatography–tandem mass spectrometry. Pharmacokinetic variables were calculated by noncompartmental analysis. The serum concentration of Tivozanib (AV951/KRN-951) at steady state after repeated p.o. administrations of a 0.2 mg/kg dose was simulated as described previously [1] Tivozanib (AV951/KRN-951) was suspended in vehicle (0.5% methyl cellulose in distilled water) and stored at 4°C. Fresh solutions were prepared weekly.[2] Experimental design. Rats inoculated with RCN‐9 cells were assigned randomly to three groups and given daily oral doses of Tivozanib (AV951/KRN-951) (1 or 3 mg/kg) or a 0.5% methylcellulose vehicle control. These treatments commenced at 4 or 14 days after tumor transplantation, and continued for 10 or 11 days, respectively. At the end of the treatment periods, the rats were killed and tumor progression was evaluated. Ascites were also collected and their volumes were measured. Each transparent window in the mesentery surrounded by fatty tissue was observed microscopically. The percentages of the mesenteric windows with a vasculature and the number of tumor nodules with or without a vasculature on the mesenteric windows were then counted. [2] In a subsequent survival study, rats inoculated with RCN‐9 cells were assigned randomly to vehicle‐treated or 1 mg/kg Tivozanib (AV951/KRN-951)‐treated groups (n = 10 per group). Separate treatments then commenced from the day of tumor inoculation or at 14 days after this transplantation. The results were plotted using Kaplan–Meier methods and the differences in survival were analyzed by log‐rank test. A P‐value of <0.05 was considered statistically significant. [2] Tumor vessel imaging. RCN‐9 cell‐inoculated rats with and without Tivozanib (AV951/KRN-951) treatment were anesthetized and injected intravenously with fluorescein isothiocyanate‐labeled dextran (molecular weight 200 000). After the animals had been killed, the mesenteries were fixed with 4% paraformaldehyde and placed on glass slides. The vasculature associated with each mesenteric window was then photographed microscopically. The number of vessel joints and paths (as vessel bifurcation characteristics), the areas and lengths of vessels (as the angiogenesis density), and the tortuosity of the vasculatures were recorded objectively and evaluated quantitatively using an angiogenesis image analyzer (Kurabo, Osaka, Japan). Four rats were used in these experiments from each group and 12–15 different fields from each animal were analyzed. [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The median Tmax of tivozanib is 10 hours, however, can range from 3 to 24 hours. A pharmacokinetic study in 8 healthy subjects revealed a Cmax and AUC for radiolabeled tivozanib of 12.1 ± 5.67 ng/mL and 1084 ± 417.0 ng·h/mL, respectively. Steady-state tivozanib concentrations are achieved at concentrations 6-7 times higher the normal dose. Tivozanib is primarily excreted in the feces. After oral ingestion of a radiolabeled 1.34 mg dose of tivozanib in healthy volunteers, 79% of the administered dose was found in the feces (with 26% unchanged) and 12% was found in the urine solely as metabolites. Tivozanib has an apparent volume of distribution (V/F) of 123 L. The apparent clearance (CL/F) of tivozanib is approximately 0.75 L/h. Metabolism / Metabolites Tivozanib is primarily metabolized by CYP3A4. After oral ingestion of a radiolabeled 1.34 mg dose of tivozanib in healthy volunteers, unchanged tivozanib accounted for 90% of the radioactive drug detected in serum. Biological Half-Life The half-life of tivozanib is about 111 hours according to prescribing information. Information from clinical studies reveals a half-life of 4-5 days. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the published preregistration clinical trials of tivozanib, rates of serum ALT or AST elevations ranged from 10% to 29%, with 1% to 4% of treated patients having elevations above 5 times the upper limit of normal (ULN). Instances of clinically apparent liver injury including deaths with hepatic failure were reported in some clinical trials, but in all cases were attributed to hepatic metastases or to other underlying liver diseases. Since its approval and more widespread clinical use, there have been no reports of clinically apparent liver injury or hepatic failure attributed to tivozanib, but it has had limited clinical use. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Protein Binding In vitro, the protein binding of tivozanib is mainly bound to albumin at a rate of ≥ 99%. |
| 参考文献 | |
| 其他信息 |
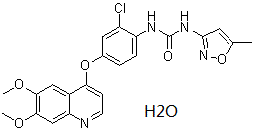
1-[2-chloro-4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-3-(5-methyl-3-isoxazolyl)urea is an aromatic ether.
Renal cell carcinoma (RCC) is responsible for 3% of cancer cases and is one of the 10 most common cancers in adults. The average age of diagnosis is between age 65 to 74. Tivozanib, also known as FOTIVDA, is a kinase inhibitor developed to treat adult patients with relapsed or refractory advanced renal cell carcinoma (RCC) after prior failed systemic therapies. It was approved on March 10, 2021 by the FDA. Marketed by Aveo Oncology, tivozanib is a promising therapy for individuals with RCC who have not been treated successfully with other therapies. Tivozanib is a Kinase Inhibitor. The mechanism of action of tivozanib is as a Tyrosine Kinase Inhibitor. Tivozanib is a small molecule multi-kinase inhibitor that is used in the treatment of relapsed or refractory renal cell carcinoma. Tivozanib is associated with transient and usually mild elevations in serum aminotransferase during therapy and but has not been linked instances of clinically apparent acute liver injury. Tivozanib is an orally bioavailable inhibitor of vascular endothelial growth factor receptors (VEGFRs) 1, 2 and 3 with potential antiangiogenic and antineoplastic activities. Tivozanib binds to and inhibits VEGFRs 1, 2 and 3, which may result in the inhibition of endothelial cell migration and proliferation, inhibition of tumor angiogenesis and tumor cell death. VEGFR tyrosine kinases, frequently overexpressed by a variety of tumor cell types, play a key role in angiogenesis. See also: Tivozanib Hydrochloride (has salt form); tivozanib hydrochloride anhydrous (is active moiety of). Drug Indication Tivozanib is approved in the USA for the treatment of relapsed or refractory renal cell carcinoma in adult patients who have undergone two or more systemic therapies. In the UK and other countries, is indicated as first line therapy of adults with advanced renal cell carcinoma (RCC) and VEGFR and mTOR pathway inhibitor-naïve patients after disease progression following one previous treatment with cytokine therapy for advanced disease. Fotivda is indicated for the first line treatment of adult patients with advanced renal cell carcinoma (RCC) and for adult patients who are VEGFR and mTOR pathway inhibitor-naïve following disease progression after one prior treatment with cytokine therapy for advanced RCC. , , Treatment of advanced renal cell carcinoma. , Mechanism of Action The VHL mutation-HIF upregulation-VEGF transcription is the main pathway implicated in the growth of renal cell carcinoma. Vascular endothelial growth factor receptors (VEGFR receptors) are important targets for tyrosine kinase inhibitors, which halt the growth of tumours. Tivozanib is a tyrosine kinase inhibitor that exerts its actions by inhibiting the phosphorylation of vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2 and VEGFR-3 and inhibits other kinases such as c-kit and platelet derived growth factor beta (PDGFR β). The above actions inhibit tumour growth and progression, treating renal cell carcinoma. Vascular endothelial growth factor (VEGF) plays a key role in tumor angiogenesis by stimulating the proangiogenic signaling of endothelial cells via activation of VEGF receptor (VEGFR) tyrosine kinases. Therefore, VEGFRs are an attractive therapeutic target for cancer treatment. In the present study, we show that a quinoline-urea derivative, KRN951, is a novel tyrosine kinase inhibitor for VEGFRs with antitumor angiogenesis and antigrowth activities. KRN951 potently inhibited VEGF-induced VEGFR-2 phosphorylation in endothelial cells at in vitro subnanomolar IC50 values (IC50 = 0.16 nmol/L). It also inhibited ligand-induced phosphorylation of platelet-derived growth factor receptor-beta (PDGFR-beta) and c-Kit (IC50 = 1.72 and 1.63 nmol/L, respectively). KRN951 blocked VEGF-dependent, but not VEGF-independent, activation of mitogen-activated protein kinases and proliferation of endothelial cells. In addition, it inhibited VEGF-mediated migration of human umbilical vein endothelial cells. Following p.o. administration to athymic rats, KRN951 decreased the microvessel density within tumor xenografts and attenuated VEGFR-2 phosphorylation levels in tumor endothelium. It also displayed antitumor activity against a wide variety of human tumor xenografts, including lung, breast, colon, ovarian, pancreas, and prostate cancer. Furthermore, dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) analysis revealed that a significant reduction in tumor vascular hyperpermeability was closely associated with the antitumor activity of KRN951. These findings suggest that KRN951 is a highly potent, p.o. active antiangiogenesis and antitumor agent and that DCE-MRI would be useful in detecting early responses to KRN951 in a clinical setting. KRN951 is currently in phase I clinical development for the treatment of patients with advanced cancer.[1] We assessed the antitumor efficacy of KRN951, a novel tyrosine kinase inhibitor of vascular endothelial growth factor receptors, using a rat colon cancer RCN-9 syngeneic model in which the tumor cells are transplanted into the peritoneal cavity of F344 rats. KRN951 treatments that commenced 4 days after tumor transplantation (day 4) significantly inhibited tumor-induced angiogenesis, the formation of tumor nodules in the mesenteric windows, and the accumulation of malignant ascites. Moreover, KRN951 treatments initiated on day 14, by which time angiogenesis and malignant ascites have already been well established, resulted in the regression of newly formed tumor vasculatures with aberrant structures and also in the apparent loss of malignant ascites by the end of the study period. Quantitative analysis of the vessel architecture on mesenteric windows revealed that KRN951 not only regressed, but also normalized the tumor-induced neovasculature. Continuous daily treatments with KRN951 significantly prolonged the survival of rats bearing both early stage and more advanced-stage tumors, compared with the vehicle-treated animals. The results of our current study thus show that KRN951 inhibits colon carcinoma progression in the peritoneal cavity by blocking tumor angiogenesis, ascites formation, and tumor spread, thereby prolonging survival. Moreover, these studies clearly demonstrate the therapeutic effects of KRN951 against established tumors in the peritoneal cavity, including the regression and normalization of the tumor neovasculature. Our findings therefore suggest that KRN951 has significant potential as a future therapeutic agent in the treatment of peritoneal cancers with ascites.[2] |
| 分子式 |
C22H21CLN4O6
|
|---|---|
| 分子量 |
472.878344297409
|
| 精确质量 |
472.114
|
| CAS号 |
682745-40-0
|
| 相关CAS号 |
Tivozanib;475108-18-0; 682745-40-0 (hydrate); 682745-41-1 (HCl hydrate)
|
| PubChem CID |
71433801
|
| 外观&性状 |
White to off-white solid powder
|
| tPSA |
109
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
631
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O(C1C=CC(NC(=O)NC2=NOC(C)=C2)=C(Cl)C=1)C1=CC=NC2C=C(OC)C(OC)=CC1=2.O
|
| InChi Key |
VTWZGSZTIGEYIC-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H19ClN4O5.H2O/c1-12-8-21(27-32-12)26-22(28)25-16-5-4-13(9-15(16)23)31-18-6-7-24-17-11-20(30-3)19(29-2)10-14(17)18;/h4-11H,1-3H3,(H2,25,26,27,28);1H2
|
| 化学名 |
1-[2-chloro-4-(6,7-dimethoxyquinolin-4-yl)oxyphenyl]-3-(5-methyl-1,2-oxazol-3-yl)urea;hydrate
|
| 别名 |
Tivozanib (hydrate); 682745-40-0; 1-[2-chloro-4-(6,7-dimethoxyquinolin-4-yl)oxyphenyl]-3-(5-methyl-1,2-oxazol-3-yl)urea;hydrate; N-[2-chloro-4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(5-methyl-3-isoxazolyl)-urea,monohydrate; Tivozanib (hydrate)?; CHEMBL4784300;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1147 mL | 10.5735 mL | 21.1470 mL | |
| 5 mM | 0.4229 mL | 2.1147 mL | 4.2294 mL | |
| 10 mM | 0.2115 mL | 1.0574 mL | 2.1147 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04987203 | Active Recruiting |
Drug: Tivozanib Drug: Nivolumab |
Renal Cell Carcinoma | AVEO Pharmaceuticals, Inc. | September 9, 2021 | Phase 3 |
| NCT01885949 | Active Recruiting |
Drug: Tivozanib Drug: Enzalutamide |
Prostate Cancer | Massachusetts General Hospital | September 3, 2013 | Phase 2 |
| NCT04645160 | Recruiting | Drug: Tivozanib | Cholangiocarcinoma Bile Duct Neoplasm |
National Cancer Institute (NCI) |
March 4, 2022 | Phase 1 Phase 2 |
| NCT06053658 | Not yet recruiting | Drug: Tivozanib Drug: Nivolumab |
Renal Cell Carcinoma | M.D. Anderson Cancer Center | January 31, 2024 | Phase 2 |
| NCT05000294 | Recruiting | Drug: Tivozanib Drug: Atezolizumab |
Bile Duct Cancer Breast Cancer |
University of Florida | November 3, 2021 | Phase 1 Phase 2 |