| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Chelating agent
|
|---|---|
| 体外研究 (In Vitro) |
屈潘特醇(PT)是一种危害环境和人类健康的有害污染物。恶臭假单胞菌S-1是一种能够利用PT作为其唯一碳源的微生物。然而,p.p putida S-1降解PT的代谢途径尚不清楚,阻碍了其优化和实际应用。在这项研究中,我们研究了p.p putida S-1脱硫过程中涉及的分解代谢网络,并鉴定了该过程中至关重要的关键基因模块。值得注意的是,propanethiol oxidoreductase (PTO)催化PT的初始降解,这是p.p putida S-1在PT上存活的关键步骤。PTO促进PT的氧化,产生H2S、H2O2和丙醛(PA)。过氧化氢酶-过氧化物酶催化H2O2转化为氧气和水,PA逐渐转化为琥珀酰辅酶a,随后用于三羧酸循环。H2S在一个全面的脱硫网络中被消化,其中硫化物-醌氧化还原酶(SQOR)主要将其转化为砜硫。转录组分析表明,硫最终可以转化为亚硫酸盐或硫酸盐,并输出到细胞外。[1]
|
| 体内研究 (In Vivo) |
通过提高PTO和SQOR基因在体内的转录水平,可以增强p.p putida S-1对屈潘特醇(PT)的降解能力。重要意义本研究研究了恶臭假单胞菌S-1中PT的分解代谢途径,这是一种能够利用PT作为唯一碳源的微生物。鉴定并表征了控制PT降解起始的关键基因,如pto和sqor。通过提高pto和sqor基因在体内的转录水平,我们成功地提高了p.p putida S-1对PT的降解效率和生长速度。这项工作不仅揭示了一种独特的PT降解途径,而且强调了在屈潘特醇污染环境的生物修复中加强微生物脱硫过程的潜力。[1]
|
| 酶活实验 |
菌株和质粒[1]
恶臭假单胞菌S-1菌株最初是从活性污泥中分离出来的,在30°C和180 rpm的条件下生长,降解 Propanethiol (PT) 。以大肠杆菌DH5α为宿主构建质粒。以大肠杆菌BL21 (DE3)为宿主进行蛋白表达。利用营养不良菌株大肠杆菌WM3064进行基因组编辑,在LB中添加2,6-二氨基戊酸(57 mg/L)进行培养。 酶活性测定[1] 根据经典的Michaelis-Menten方程分析了PTO和SQOR的稳态动力学。将PTO和SQOR的蛋白浓度稀释至100µM进行催化反应。FAD和CoQ1的浓度也设置为100µM。在一定的底物浓度下进行催化反应,并计算生成MM图的初始速率。使用graphpad Prism version 8软件对采集的数据进行拟合。 |
| 参考文献 | |
| 其他信息 |
VOSC degradation micrograms are often highly substrate specific. Previously reported studies of thiol biodegradation mainly focused on the degradation of methanethiol; a detailed characterization of the comprehensive catabolism pathway, however, has not been conducted. This study provides insights into the fine-tuned propanethiol catabolic mechanism network in Pseudomonas putida S-1 and identified critical gene modules accounting for the initiation of PT desulfurization. Based on the experimental observations made, the PT catabolism in P. putida S-1 was initiated by PTO (S1GL003403), and the reaction products H2O2, H2S, and propionaldehyde were then catalyzed by SQOR (S1GL000007 and S1GL003435) and the genes downstream. In the BRENDA Enzyme Databse (https://www.brenda-enzymes.org/index.php), oxidases with thiol as the substrates are categorized into two groups, thiol oxidase (EC 1.8.3.2) and methanethiol oxidase (MTO) (EC 1.8.3.4). Thiol oxidase converts thiols to the corresponding thioether, while MTO is restricted to one substrate, methanethiol. For example, the first MTO enzyme reported was isolated from the bacterium Hyphomicrobium sp. VS, and the Km value of this protein is around 0.3 µM. The MTO from human beings catalyzes methanethiol to sulfide in a similar mechanism and has an apparent Km of 1.8 nM, and this low Km value could be contributing to the prevention of methanethiol toxicity in the human body. Although these MTO enzymes catalyze methanethiol to sulfide with relatively high efficiency, their substrate spectrum is narrow. Propanethiol, for example, could not be catalyzed by them. The substrate specificity of PTO exhibits a high degree of selectivity. It was observed that the bacterium P. putida S-1, from which PTO was isolated, was unable to thrive solely on MT as a carbon source. If PTO were capable of degrading MT, one would expect the bacterium to utilize the resulting products as a nutritional source. The limited range of substrates for MTO or PTO implies that oxidases acting on thiols as substrates are likely to display pronounced substrate specificity. The discovery of PTO addresses a significant gap in the existing enzyme repertoire, suggesting its potential application in the bioremediation of PT pollution.
[1]
After the initial desulfurization step, SQOR genes S1GL000007 and S1GL003435 conduct the conversion of H2S to sulfane sulfur, GSSH, or thiosulfate (Fig. S8). Whole-genome sequencing has also annotated three genes potentially encoded for cysteine synthase that can catalyze sulfide to cysteine, but all of them were downregulated under PT induction (Table S5). Considering cysteine was reported to form reactive oxygen species during oxidization that can damage DNA and lead to mutations or cell death, the generation of cysteine might not be the key step for sulfur circulation in P. putida S-1, although cysteine could serve as the carbon source for the bacteria. GSSH, the product of SQOR, could be oxidized by PDO to sulfite, which could be oxidized to sulfate naturally or by SDH, and some of these genes (e.g., S1GL000006) were upregulated in P. putida S-1 with PT induction (Table S5). Sulfate could be transported out of P. putida S-1 through a sulfite exporter (TAUE) or reduced to sulfide by sulfite reductase (SRD). As PT exhibits limited solubility in water, it could form disulfide in the environment and enter the cell. Unlike tauE, the only srd gene was significantly downregulated in P. putida S-1 (Table S5). Sulfite can be converted to thiosulfate by a thiosulfate sulfurtransferase, and thiosulfate can be further transferred to tetrathionate. However, the SfnB family sulfur acquisition oxidoreductase (SFNB) that catalyzes this reaction was largely downregulated, suggesting tetrathionate is not a favorable metabolite during PT catabolism. Propionaldehyde generated by PTO could be converted to propionate by aldehyde dehydrogenase. The propionate is converted to propionyl-coenzyme A (CoA) by propionate CoA-transferase. Finally, propionyl-CoA is catalyzed to succinyl-CoA by propionyl-CoA:succinyl-CoA transferase and enters the tricarboxylic acid cycle (Fig. S8). [1] In conclusion, the critical gene modules S1GL003403 (pto) and S1GL003435 (sqor) were successfully found to be responsible for the initial catabolism process of the PT catabolism pathway in Pseudomonas putida S-1. When increasing the transcription level of pto and sqor genes, PT degradation capacity of P. putida S-1 can be significantly enhanced. These findings put new insight of and suggest more strategy to enhance the microbial desulfurization process.[1] This work investigated the PT catabolism pathway in Pseudomonas putida S-1, a microorganism capable of utilizing PT as the sole carbon source. Critical genes that control the initiation of PT degradation were identified and characterized, such as pto and sqor. By increasing the transcription level of pto and sqor genes in vivo, we have successfully enhanced the PT degradation efficiency and growth rate of P. putida S-1. This work does not only reveal a unique PT degradation pathway but also highlights the potential of enhancing the microbial desulfurization process in the bioremediation of thiol-contaminated environment. |
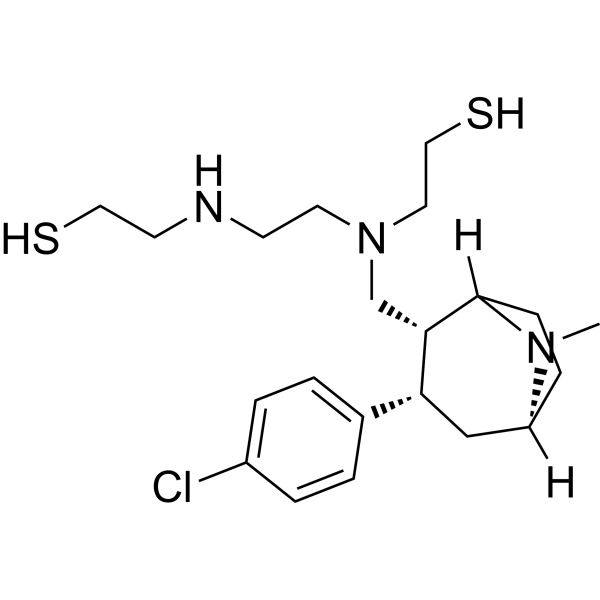
| 分子式 |
C21H34CLN3S2
|
|---|---|
| 分子量 |
428.094
|
| 精确质量 |
427.188
|
| 元素分析 |
C, 58.92; H, 8.01; Cl, 8.28; N, 9.82; S, 14.98
|
| CAS号 |
189950-11-6
|
| 相关CAS号 |
675825-78-2 (HCl); 189950-11-6
|
| PubChem CID |
10670183
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.143g/cm3
|
| 沸点 |
534.622ºC at 760 mmHg
|
| 闪点 |
277.129ºC
|
| LogP |
3.986
|
| tPSA |
96.11
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
434
|
| 定义原子立体中心数目 |
4
|
| SMILES |
SCCNCCN(CC1C(C2C=CC(Cl)=CC=2)CC2CCC1N2C)CCS
|
| InChi Key |
HZLFSOZSLFKJKA-JSXRDJHFSA-N
|
| InChi Code |
InChI=1S/C21H34ClN3S2/c1-24-18-6-7-21(24)20(15-25(11-13-27)10-8-23-9-12-26)19(14-18)16-2-4-17(22)5-3-16/h2-5,18-21,23,26-27H,6-15H2,1H3/t18-,19+,20-,21+/m0/s1
|
| 化学名 |
2-[2-[[(1R,2R,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octan-2-yl]methyl-(2-sulfanylethyl)amino]ethylamino]ethanethiol
|
| 别名 |
NC 100697; NC-100697; Tropantiol; 189950-11-6; Tropantiol [USAN:INN]; NC100697; UNII-7844H41L5Z; 7844H41L5Z; TROPANTIOL [INN]; TROPANTIOL [USAN];
NC100697
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3360 mL | 11.6798 mL | 23.3596 mL | |
| 5 mM | 0.4672 mL | 2.3360 mL | 4.6719 mL | |
| 10 mM | 0.2336 mL | 1.1680 mL | 2.3360 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。