| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 靶点 |
Tau protein aggregation (Ki = 0.12 μM)
|
|---|---|
| 体外研究 (In Vitro) |
除了降低 tau 和 p-tau 表达水平外,无色亚甲基蓝(100 nM,48 h)甲磺酸盐还消除了 Aβ25-35 对 β-A 和腺苷 A1R 表达水平的鼓舞作用 [2]。
TauRx Therapeutics(新加坡,新加坡共和国)正在开发一种稳定的、还原形式的MTC,TRx 0237(LMTX™)。一项体外研究表明,TRx 0237在0.16μM的浓度下具有破坏从AD脑组织分离的PHF的能力。该值与MT(0.16μM)的结果相同[3]。 |
| 体内研究 (In Vivo) |
在两种过表达不同人类tau蛋白构建体的新型小鼠模型(L1和L66)中比较了MTC和TRx 0237(5-75mg/kg口服3-8周)的体内效果。MTC和TRx 0.237在L1的空间问题解决水迷宫任务中剂量依赖性地挽救了学习障碍并恢复了行为灵活性(MTC的最小有效剂量为35mg MT/kg,TRx 0237为9mg MT/kg),在L66的纠正运动学习中剂量依赖于4 mg MT/kg)。这两种化合物都减少了tau反应性神经元的数量,特别是在L1的海马和内嗅皮层,在L66中更为普遍[3]
在最近完成的测试tau聚集抑制剂隐色甲硫氨酸双(氢氟烷磺酸盐)(LMTM)的3期试验中,我们发现,根据患者是将LMTM作为单一疗法还是作为对症治疗的补充,治疗反应存在显著差异。[4] 方法[4] 我们研究了单独使用LMTM或在LMTM治疗表达构成AD缠结丝的短tau片段的tau转基因小鼠之前使用慢性利凡斯的明的效果。我们测量了乙酰胆碱水平、突触体谷氨酸释放、突触蛋白、线粒体复合物IV活性、tau病理学和胆碱乙酰转移酶(ChAT)免疫反应性。 结果[4] 单独给予LMTM可增加海马乙酰胆碱(ACh)水平、突触体制剂中谷氨酸的释放、多个脑区突触素水平和线粒体复合物IV活性,减少tau病理,部分恢复基底前脑的ChAT免疫反应性,并逆转空间学习缺陷。除了减少tau聚集病理外,发现利凡斯的明慢性预处理可以减少或消除几乎所有这些影响。LMTM对野生型小鼠海马ACh和突触素水平的影响也降低了。 结论[4] 胆碱酯酶抑制剂对LMTM药理活性的干扰可以在tau转基因小鼠模型中复制,在较小程度上也可以在野生型小鼠中复制。长期使用有症状的药物进行预处理会改变不同递质系统和细胞隔室在多个脑功能水平上对LMTM的广泛脑反应。因此,负相互作用没有单一的位点。相反,胆碱酯酶功能降低引起的慢性神经元激活在多个神经元系统中产生代偿性稳态下调。这减少了与tau聚集病理减少相关的LMTM的广泛治疗反应。由于干扰是由对先前对症治疗的稳态反应决定的,因此无论预期的治疗靶点或作用方式如何,作为现有对症治疗的附加药物,很可能会对其他测试药物产生类似的干扰。目前的研究结果概述了关键结果,这些结果现在提供了一个工作模型来解释对症治疗的干扰。 |
| 细胞实验 |
蛋白质印迹分析[2]
细胞类型:人类 SH-SY5Y 细胞系。 测试浓度:100 nM。 孵化持续时间:48小时。 实验结果: Aβ25-35和TRx 0237共同处理显着逆转了Aβ25-35对tau、p-tau、orexin A和腺苷A1R表达的促进作用。 SH-SY5Y细胞用或不用tau抑制剂TRx 0237处理。SH-SY5Y细胞被分为阴性对照(SH-SY5Y)和Aβ25-35(SH-SI5Y+Aβ25-35)或TRx 0237(SH-SY-Aβ25-35+TRx 0237]。将SH-SY5Y细胞(1×105个细胞/孔)接种在6孔板中,用载体Aβ25-35或TRx 0237转染48小时。使用前,将Aβ25-35,在无菌生理盐水中稀释至0.5mM的浓度,并在37°C下保持7天,以对肽进行预老化(24)。将老化的Aβ溶液稀释至40µM以供使用[2]。 |
| 动物实验 |
Processing of specimen batches[1]
Extraction specimens were processed in batches consisting of six calibration standards (10.0 to 20,000 ng/mL), QC samples, extract storage stability samples, and control (blank) samples. Following processing, each standard was split into two aliquots, one of which was run at the beginning and the other at the end of each analytical run. A total of 48 samples/standards were processed in each run. Analysis preparation for each animal sample consisted of two separate extractions, one extraction for the quantitation of excreted methylene blue, and the second extraction for the quantitation of leucomethylene blue/TRx 0237. In order to prepare samples for extraction of methylene blue, 200 lJL of 1M NaCl solution and 100 IJL of Basic Blue 3 internal standard solution were added to 0.5 mL of urine in polypropylene tubes. Tube contents were vortex mixed gently. A 4-mL aliquot of 1,2-dichloroethane was added to each tube, and the contents were shaken for 15 min and then centrifuged for 5 min with a table-top centrifuge. The 1,2-dichloroethane layer was transferred to a clean 4-mL polypropylene vial using a polyethylene pipette and evaporated to dryness with a Savant Speedvac sample concentrator set to medium heat. A 200-~L aliquot of 0.1% trifluoroacetic acid solution and 100 ~L of acetonitrile were added to each vial, and the contents were sonicated for 6 min and transferred to a polypropylene autosampler vial insert. A second extraction was then performed to remove the leucomethylene blue/TRx 0237 from the samples following conversion of the leucomethylene blue present to methylene blue. To convert the leucomethylene blue to methylene blue, 1001JL of 1N HC1 was added to the tubes. The tubes were immersed in boiling water for 20 min and allowed to cool to room temperature. The methylene blue which formed from oxidation of leucomethylene blue was then extracted and prepared for analysis using the same technique used for the original sample extraction. Acetylcholine Measurement in Hippocampus[4] Animals were treated with LMTM (leuco-methylthioninium bis (hydromethane-sulfonate) (5 mg/kg/day for 2 weeks, gavage) after prior treatment for 2 weeks with or without rivastigmine (0.5 mg/kg/day subcutaneous Alzet minipump). Levels of ACh were measured in the hippocampus via indwelling microdialysis probes and HPLC analysis of the extracellular fluid. After the experiment, brains were harvested and histologically assessed for correct cannula placement. The treatment schedule used to study the negative interaction between symptomatic treatments and LMTM was designed to model the clinical situation in which subjects are first treated chronically with a cholinesterase inhibitor or memantine before receiving LMTM Fig. (1). After five weeks of daily gavaging with vehicle or rivastigmine, combination treatment proceeded in some groups while others received only LMTM monotherapy. [4] Wild-type and L1 female mice (n = 7-16 for each group) were pre-treated with rivastigmine (0.1 or 0.5 mg/kg/day) or vehicle for 5 weeks by gavage. For the following 6 weeks, LMTM (5 and 15 mg/kg) was added to this daily treatment regime, also administered by gavage Fig. (1). Animals were then sacrificed for immunohistochemical and other tissue analyses, as described in a study [36]. Although 5 mg/kg/day in mice corresponds approximately to 8 mg/day in humans in terms of Cmax levels of parent MT in plasma, this dose is at the threshold for effects on pathology and behaviour. The higher dose of 15 mg/kg/day is generally required for LMTM to be fully effective in the L1 mouse model. This may relate to the much shorter half-life of MT in mice (4 hours) compared to humans (37 hours in elderly humans). |
| 药代性质 (ADME/PK) |
TRx0237 is claimed to have a better pharmacokinetic and tolerability profile than MTC, but not convincing evidences have been provided to support this. The better oral absorption of TRx0237 compared to MTC in the presence of food showed in healthy volunteers did not translate in higher CNS levels since drug brain levels in minipigs are almost identical after 33 mg/kg (about 5 μM) of MT or TRx0237. On the other hand, no data on TRx0237 CSF concentrations in humans are available. No robust data on safety and tolerability of TRx0237 in humans are available to make direct comparison with MTC. Comparative in vitro data showed a therapeutic index (ratio of LD50/EC50) of 92 for LMT-dihydrobromide and 179 for LMT-dihydromesylate compared to value of 110 for MTC. We believe that these in vitro differences were not so dramatic to necessarily translating in pharmacological or clinical differences. In terms of efficacy, pharmacological studies in transgenic mouse tauopathy models did not show dramatic differences between the two compounds. Indeed, a dose of 45 mg/kg of MTC or TRx0237 produced identical behavioral effects.[3]
|
| 毒性/毒理 (Toxicokinetics/TK) |
A safety and tolerability study of TRx0237 (250 mg/day for 4 weeks) in nine patients with mild-to-moderate AD began in September 2012 but it was terminated in April 2013, reportedly for administrative reasons (ClinicalTrials.gov Identifier: NCT01626391) (Table 1). Three Phase III placebo-controlled studies with TRx0237 are ongoing (Table 1). The first study is evaluating the 200-mg/day dose in 700 patients with a diagnosis of either all-cause dementia and probable AD and adopted the cognitive ADAS-Cog 11 scale and the clinical Alzheimer’s Disease Cooperative Study – Clinical Global Impression of Change (ADCS-CGIC) scale as primary efficacy variables (ClinicalTrials.gov Identifier: NCT01689233). The second study is evaluating the doses of 150 and 250 mg/day in 833 patients with mild-to-moderate AD and is using the ADAS-Cog 11 and ADCS-CGIC as primary endpoints (ClinicalTrials.gov Identifier: NCT01689246). The third Phase III trial is evaluating the 200 mg/day dose in 220 patients affected by the behavioral variant of frontotemporal dementia (bvFTD) (ClinicalTrials.gov Identifier: NCT01626378). This trial adopted a modified version of the ADCS-CGIC scale as measure of clinical efficacy and the revised Addenbrooke’s Cognitive Examination as cognitive measure. Finally, an open-label extension study in subjects who have completed participation in a Phase II or Phase III trials with TRx0237 is evaluating the long-term safety of the compound (ClinicalTrials.gov Identifier: NCT02245568) (Table 1). With the hope of maintaining blinding, the Phase III studies are using ‘active placebo’ tablets that include 4 mg of TRx0237 as a urinary and fecal colorant. Overall, these Phase III trials are recruiting 1753 patients at 250 centers in 22 countries and results are expected in the first half of 2016 for one of these trials (ClinicalTrials.gov Identifier: NCT01689246) and in the second half of 2016 for the other two studies (ClinicalTrials.gov Identifiers: NCT01689233 and NCT01626378).[3]
|
| 参考文献 |
|
| 其他信息 |
HYDROMETHYLTHIONINE MESYLATE is a small molecule drug with a maximum clinical trial phase of III (across all indications) and has 2 investigational indications.
A liquid chromatographic method for the determination of methylene blue and leucomethylene blue in male and female Fischer 344 rat urine and male and female B6C3F1 mouse urine was developed for use in supporting toxicokinetic studies, validated, and used to analyze urine samples for a preliminary dose level range-finding study. The method was validated for a concentration range of 10.0 to 20,000 ng/mL in urine. Samples up to 75,000 ng/mL demonstrated good recoveries when diluted into the range of the calibration curve. Six sets of calibration standards were prepared in F344 male rat urine for analysis to demonstrate reproducibility and ruggedness. The stability of sample extracts was determined under various storage conditions. During the course of analyses of the animal samples, a possible metabolic process was observed. Although Azure B is a significant impurity in methylene blue trihydrate, the amount of Azure B seen in urine samples collected from rodents dosed with methylene blue trihydrate is significantly greater than the amount seen in rodent urine spiked directly with methylene blue. This observation suggests possible N-demethylation of the methylene blue as a metabolic transformation to Azure B.[1] Sleep disorder is confirmed as a core component of Alzheimer's disease (AD), while the accumulation of amyloid β (Aβ) in brain tissue is an important pathological feature of AD. However, how Aβ affects AD‑associated sleep disorder is not yet well understood. In the present study, experiments on animal and cell models were performed to detect the association between sleep disorder and Aβ. It was observed that Aβ25‑35 administration significantly decreased non‑rapid eye movement sleep, while it increased wakefulness in mice. In addition, reverse transcription‑quantitative polymerase chain reaction and western blot analysis revealed that the expression levels of tau, p‑tau, orexin A and orexin neurons express adenosine A1 receptor (A1R) were markedly upregulated in the brain tissue of AD mice compared with that in samples obtained from control mice. Furthermore, the in vitro study revealed that the expression levels of tau, p‑tau, orexin A and adenosine A1R were also significantly increased in human neuroblastoma SH‑SY5Y cells treated with Aβ25‑35 as compared with the control cells. In addition, the tau inhibitor TRx 0237 significantly reversed the promoting effects of Aβ25‑35 on tau, p‑tau, orexin A and adenosine A1R expression levels, and adenosine A1R or orexin A knockdown also inhibited tau and p‑tau expression levels mediated by Aβ25‑35 in AD. These results indicate that Aβ and tau may be considered as novel biomarkers of sleep disorder in AD pathology, and that they function by regulating the expression levels of orexin A and adenosine A1R. [2] In the last 10 years, several clinical trials with anti-Aβ agents failed, challenging the hypothesis that Aβ accumulation is the initiating event in the pathological AD cascade, and underscoring the need for novel therapeutic approaches and targets. Among TAIs, MT belongs to a class of diaminophenothiazines that have TAI activity in vitro. MTC, in which MT is dosed as the oxidized form MT+, was investigated in an exploratory Phase II dose-ranging double-blind clinical trial in 321 patients with mild-to-moderate AD. The minimum effective dose was identified as 138 mg MT/day at both clinical and molecular imaging endpoints at 24 weeks. Treatment at this dose was found to prevent the decline in regional cerebral blood flow, particularly in medial temporal lobe structures and temporoparietal regions. Given that the delivery of the highest dose of MT was impaired due to dose-dependent dissolution and absorption limitations, four Phase I studies and two preclinical in vitro and in vivo studies were required to get to the bottom of the bioavailability limitations of the form of MT tested in the Phase II trial, setting out the basis for proceeding into Phase III trials with TRx0237 for AD treatment. TRx0237 is claimed to have a better pharmacokinetic and tolerability profile than MTC, but not convincing evidences have been provided to support this. The better oral absorption of TRx0237 compared to MTC in the presence of food showed in healthy volunteers did not translate in higher CNS levels since drug brain levels in minipigs are almost identical after 33 mg/kg (about 5 μM) of MT or TRx0237. On the other hand, no data on TRx0237 CSF concentrations in humans are available. No robust data on safety and tolerability of TRx0237 in humans are available to make direct comparison with MTC. Comparative in vitro data showed a therapeutic index (ratio of LD50/EC50) of 92 for LMT-dihydrobromide and 179 for LMT-dihydromesylate compared to value of 110 for MTC. We believe that these in vitro differences were not so dramatic to necessarily translating in pharmacological or clinical differences. In terms of efficacy, pharmacological studies in transgenic mouse tauopathy models did not show dramatic differences between the two compounds. Indeed, a dose of 45 mg/kg of MTC or TRx0237 produced identical behavioral effects [3] |
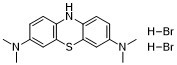
| 分子式 |
C16H21BR2N3S
|
|---|---|
| 分子量 |
447.233
|
| 精确质量 |
444.982
|
| 元素分析 |
C, 42.97; H, 4.73; Br, 35.73; N, 9.40; S, 7.17
|
| CAS号 |
951131-15-0
|
| 相关CAS号 |
613-11-6;1236208-20-0 (mesylate);61-73-4 (chloride);951131-15-0 (HBr);
|
| PubChem CID |
23651551
|
| 外观&性状 |
Solid powder
|
| tPSA |
43.8
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
304
|
| 定义原子立体中心数目 |
0
|
| SMILES |
Br.Br.S1C2C=C(C=CC=2NC2C=CC(=CC1=2)N(C)C)N(C)C
|
| InChi Key |
JAUPSVVTGFBHTN-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H19N3S.2BrH/c1-18(2)11-5-7-13-15(9-11)20-16-10-12(19(3)4)6-8-14(16)17-13/h5-10,17H,1-4H32*1H
|
| 化学名 |
N3,N3,N7,N7-Tetramethyl-10H-phenothiazine-3,7-diamine dihydrobromide
|
| 别名 |
TRX-0237; Leucomethylene Blue dihydrobromide; TRX-0237 dihydrobromide; TRX0237; TRX 0237; 951131-15-0; Leucomethylene Blue dihydrobromide; TRx0237; E79ZM68IOZ; Leukomethylene Blue dihydrobromide; Hydromethylthionine (dihydrobromide); Reduced methylene Blue dihydrobromide; N3,N3,N7,N7-Tetramethyl-10H-phenothiazine-3,7-diamine dihydrobromide; TRX-0237 HBr; Leukomethylene Blue dihydrobromide; Reduced methylene Blue dihydrobromide; Hydromethylthionine HBr
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2360 mL | 11.1799 mL | 22.3599 mL | |
| 5 mM | 0.4472 mL | 2.2360 mL | 4.4720 mL | |
| 10 mM | 0.2236 mL | 1.1180 mL | 2.2360 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。