| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
CDK9/cyc T2 (Ki = 0.626 nM); CDK9/CycT1 (Ki = 1.68 nM); CDK6/cycD1 (Ki = 2.92 nM); CDK4/Cyc D1 (Ki = 3.96 nM); CDK1/cycB (Ki = 5.4 nM); CDK1/cyc A (Ki = 9.1 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Voruciclib(0.5-5 µM;6 小时)显示 ABC 和 GCB 亚型中 MCL-1 的靶向下调[1]。细胞活力测定[1] 细胞系:U2932、RIVA、OCI-LY10 细胞(ABC 亚型)、NU-DHL-1、SU-DHL-4、SU-DHL-6 细胞(GCB 亚型) 浓度:0.5 µM,1 µM、2 µM、3 µM、4 µM、5 µM 孵育时间:6 小时 结果:在 ABC 和 GCB 亚型中均显示 MCL-1 的靶向下调。
|
| 体内研究 (In Vivo) |
盐酸 Voruciclib(200 mpk;口服强饲)与 Venetoclax(U2932、RIVA、SU-DHL-4 和 NU-DHL-1 中分别为 10 mpk、1 mpk、50 mpk、25 mpk)组合可增强肿瘤生长抑制作用在 U2932、RIVA、SU-DHL-4(每周 6 天,持续 4 周)和 NU-DHL-1 模型(每周 5 天,持续 3 周)DLBCL 模型中,与单独使用任一药物相比[1]。动物模型:ABC 亚型(U2932、RIVA、OCI-LY10)、GCB 亚型(SU-DHL-4、NU-DHL-1)异种移植到雌性 NOD.CB17-Prkdcscid/NCrHsd 小鼠中剂量:200 mpk 给药方式:口服强饲; U2932、RIVA、SU-DHL-4(每周 6 天,持续 4 周)、OCI-LY10(每周 6 天,持续 2 周)、NU-DHL-1(每周 5 天,持续 3 周) 结果:肿瘤增强除 OCI-LY10 模型外,U2932、RIVA、SU-DHL-4 和 NU-DHL-1 模型中的生长抑制。
|
| 酶活实验 |
生物信息学研究进展[1][br]
反应生物学公司测定了48种激酶对盐酸voruciclib的敏感性等级,以放射性γ-33P-ATP为磷酸供体,采用过滤结合法测定激酶活性。各激酶的ATP浓度接近Km值。对于每个激酶,从试验品的10点浓度曲线计算IC50值并转换为Ki值。本文研究的48种激酶在先前的筛选实验中被确定为最有希望的靶标候选。
|
| 细胞实验 |
细胞系:U2932、RIVA、OCI-LY10 细胞(ABC 亚型)、NU-DHL-1、SU-DHL-4、SU-DHL-6 细胞(GCB 亚型)
浓度:0.5 µM、1 µM、2 µM、3 µM、4 µM、5 µM 孵育时间:6 小时 结果:在 ABC 和 GCB 亚型中均显示 MCL-1 的靶向下调。 |
| 动物实验 |
ABC subtypes (U2932, RIVA, OCI-LY10), GCB subtypes (SU-DHL-4, NU-DHL-1) xenografted in Female NOD.CB17-Prkdcscid/NCrHsd mice
200 mpk Oral gavage; U2932, RIVA, SU-DHL-4 (six days per week for 4 weeks), OCI-LY10 (six days per week for 2 weeks), NU-DHL-1 (five days per week for 3 weeks) For systemic drug efficacy studies, mice were enrolled when tumors reached an average volume of 150–200 mm3. Assignment to treatment groups was carried out via stratified randomization. Tumor dimensions were measured twice a week using digital calipers along with recording of body weight. Tumor volume was calculated using formula V = π/6 (length x width x height) in mm3 . Animals were removed from the study when either any one of the three measured dimensions of the tumor exceeded 2 cm, volume exceeded 2000 mm3, ulceration was observed or if body weight loss greater than 20% was recorded. Drug formulations: voruciclib was formulated in 0.1% methylcellulose and venetoclax in 60% phosal 50, 30% PEG 400, 10% ethanol, both administered orally. Control cohorts were administered vehicles of both drugs, and each single agent cohort was administered the vehicle of the other drug. Depending on the assigned treatment, venetoclax (or its vehicle) was followed by voruciclib (or its vehicle) with 30 mins in between administrations to allow for gastric clearance as per veterinary recommendation. Model specific dosing regimens: U2932: venetoclax at 10 mpk twice a week, voruciclib 200 mpk six days per week for a total duration of 4 weeks; RIVA: venetoclax at 1 mpk twice a week, voruciclib 200 mpk six days per week for a total duration of four weeks; OCI-LY10: venetoclax at 25 mpk twice a week, voruciclib 200 mpk six days per week for a total duration of two weeks; NU-DHL-1: venetoclax at 50 mpk once a week, voruciclib at 200 mpk five days per week for a total duration of three weeks; SU-DHL-4: venetoclax at 25 mpk and voruciclib 200 mpk both six days per week for a total duration of four weeks. The venetoclax + voruciclib combination treatment cohort for each model received both drugs at the same doses and frequencies as the corresponding single agents. |
| 参考文献 | |
| 其他信息 |
Voruciclib is under investigation in clinical trial NCT03547115 (A Phase 1 Study of Voruciclib in Subjects With B-Cell Malignancies or AML).
Voruciclib is a cyclin-dependent kinase (CDK) inhibitor with potential antineoplastic activity. Upon administration, voruciclib selectively inhibits cyclin-dependent kinase 4 (CDK4) and 6 (CDK6). This inhibits retinoblastoma (Rb) protein phosphorylation early in the G1 phase, which prevents CDK-mediated G1-S phase transition and leads to cell cycle arrest. This suppresses DNA replication and decreases tumor cell proliferation. CDK4 and 6 are serine/threonine kinases that are upregulated in many tumor cell types and play a key role in the regulation of cell cycle progression. |
| 分子式 |
C22H19CLF3NO5
|
|---|---|
| 分子量 |
469.8412
|
| 精确质量 |
469.09
|
| 元素分析 |
C, 56.24; H, 4.08; Cl, 7.55; F, 12.13; N, 2.98; O, 17.03
|
| CAS号 |
1000023-04-0
|
| 相关CAS号 |
Voruciclib hydrochloride;1000023-05-1;(2S,3R)-Voruciclib hydrochloride;rel-(2S,3R)-Voruciclib;1253731-24-6; 1000023-04-0; 2505206-37-9 (malonate)
|
| PubChem CID |
67409219
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| LogP |
4.261
|
| tPSA |
94.14
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
750
|
| 定义原子立体中心数目 |
2
|
| SMILES |
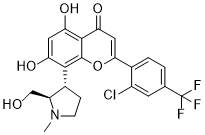
ClC1C=C(C(F)(F)F)C=CC=1C1=CC(C2=C(C=C(C(=C2O1)[C@@H]1CCN(C)[C@@H]1CO)O)O)=O
|
| InChi Key |
MRPGRAKIAJJGMM-OCCSQVGLSA-N
|
| InChi Code |
InChI=1S/C22H19ClF3NO5/c1-27-5-4-12(14(27)9-28)19-15(29)7-16(30)20-17(31)8-18(32-21(19)20)11-3-2-10(6-13(11)23)22(24,25)26/h2-3,6-8,12,14,28-30H,4-5,9H2,1H3/t12-,14+/m1/s1
|
| 化学名 |
2-[2-chloro-4-(trifluoromethyl)phenyl]-5,7-dihydroxy-8-[(2R,3S)-2-(hydroxymethyl)-1-methylpyrrolidin-3-yl]chromen-4-one
|
| 别名 |
Voruciclib; 1000023-04-0; Voruciclib [INN]; P1446A-05; 2-[2-chloro-4-(trifluoromethyl)phenyl]-5,7-dihydroxy-8-[(2R,3S)-2-(hydroxymethyl)-1-methylpyrrolidin-3-yl]chromen-4-one; W66XP666AM; CHEMBL3905910; P-1446;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~50 mg/mL (~106.4 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1284 mL | 10.6419 mL | 21.2838 mL | |
| 5 mM | 0.4257 mL | 2.1284 mL | 4.2568 mL | |
| 10 mM | 0.2128 mL | 1.0642 mL | 2.1284 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03547115 | Recruiting | Drug: voruciclib monotherapy Drug: voruciclib and venetoclax |
Follicular Lymphoma (FL) Acute Myeloid Leukemia (AML) |
MEI Pharma, Inc. | May 31, 2018 | Phase 1 |
|
|
|