| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 靶点 |
Human Endogenous Metabolite; NF-κB; Mitochondrial bioenergetics; HIV-1
|
|---|---|
| 体外研究 (In Vitro) |
HIV-1 的长末端重复序列 (LTR) 是 NF-κB 等细胞转录因子的靶标,当整合到宿主 DNA 中时,可作为病毒基因组的启动子增强子[1]。二硫醇化合物α-硫辛酸(α-lipoic acid, ALA)是一种天然存在的物质,对线粒体生物能至关重要。通过激活 SIRT1/LKB1/AMPK 通路来控制转录因子 SREBP-1、FoxO1 和 Nrf2 及其下游脂肪生成靶标,α-硫辛酸可减少肝脏中的脂质积累。 α-硫辛酸(250、500和1000 μM)处理后,HepG2细胞中NAD+/NADH比值显着升高(P<0.05或P<0.01)。在 HepG2 细胞中,α-硫辛酸(50、125、250 和 500 μM)处理会增加 SIRT1 活性。在 HepG2 细胞中,α-硫辛酸(50、125、250、500 和 1000 μM)以剂量依赖性方式增加 AMPK 和乙酰辅酶A羧化酶 (ACC) 磷酸化[1]。
肝细胞癌(HCC)是最常见的癌症,也是成人死亡的主要原因。目前治疗HCC的方法存在耐药性和预后不良的问题;因此,迫切需要新的治疗药物。植物化学物质已被提出用于治疗一系列癌症。其中,lipoic acid/α-硫辛酸(α-LA)是一种天然合成的抗氧化剂,存在于各种膳食动植物来源中,可防止正常细胞中氧化介导的细胞死亡,同时诱导几种癌症细胞系凋亡。以前,我们证明了用α-LA治疗肝癌细胞会诱导凋亡,在此之前会产生活性氧(ROS)和激活p53蛋白,p53蛋白是线粒体介导的凋亡的已知诱导剂。多项研究表明,ROS诱导的细胞凋亡与内质网(ER)应激和未折叠蛋白反应(UPR)激活有关。在此,我们通过基因表达谱分析研究了α-LA诱导的肝癌细胞系凋亡是否是ER应激和UPR介导的。α-LA治疗后,UPR和ER应激途径的上调最为明显。这一发现已通过ER和UPR相关蛋白的表达分析得到证实,为更好地理解α-LA对肝癌细胞抗肿瘤作用背后的分子机制提供了依据。[4] 分别通过硫辛酸(LA)、二硫代二丙酸(DA)和己二酸(AA)修饰的TrxR抑制剂(CPUL1)构建了三种自组装纳米聚集体(CPUL1-LA NA、CPUL1-DA NA和CPUL1-AA NA)。DLS、TEM、UV-vis、荧光、1H NMR、ITC和MTT分析的测量验证了含二硫化物的CPUL1-LA NA和CPUL1-DA NA在水溶液中自发组装无载体纳米颗粒,其具有高药物含量、优异的稳定性、对HUH7肝癌细胞的细胞毒性改善,以及对L02正常细胞的低细胞毒性,具有潜在的生物安全性。相比之下,无二硫化物的CPUL1-AA NA在48小时后发生聚集和沉淀,在水溶液中表现出明显的不稳定性。因此,二硫化物单元似乎对构建可控和稳定的纳米聚集体至关重要。在测量TrxR/NADPH和GSH/GR/NADPH对纳米聚集体的减少时,证实LA的环状二硫化物和DA的线性二硫化物赋予纳米聚集体靶向能力,使其对TrxR的特异性反应超过GSH。此外,通过流式细胞术、荧光图像和CLSM的测试,CPUL1-LA-NA和CPUL1-DA-NA都表现出更快的细胞摄取特征,可被癌症细胞内部化,并可产生比游离CPUL1更丰富的ROS来诱导细胞凋亡,从而显著提高体外对HUH7细胞的抗肿瘤功效[5]。 |
| 体内研究 (In Vivo) |
为了引起非酒精性脂肪肝(NAFLD),C57BL/6J小鼠被分为四组,并喂食高脂肪饮食(HFD)24周。然后每天给每组施用α-硫辛酸。然后在长期 HFD 喂养的小鼠中检查 α-硫辛酸/lipoic acid对肝脏脂质积累的影响。接受100mg/kg或200mg/kg的α-硫辛酸后,小鼠内脏脂肪量显着减少。此外,α-硫辛酸(100 mg/kg 或 200 mg/kg)治疗可降低食欲并导致体重显着减轻(均 P0.05)[1]。
了解α-硫辛酸补充剂在体内和体外对非酒精性脂肪肝疾病具有保护作用的机制,可能会导致预防肝脂肪变性的目标。雄性C57BL/6J小鼠被喂食正常饮食、高脂肪饮食或补充α-硫辛酸的高脂肪饮食24周。将HepG2细胞与正常培养基、棕榈酸酯或α-硫辛酸一起孵育。测量了降脂作用。分别通过Western blot、免疫沉淀和免疫荧光分析蛋白质表达和分布。我们发现α-硫辛酸通过肝激酶B1增强去乙酰化酶1的活性,并刺激AMP激活的蛋白激酶。通过激活去乙酰化酶1/肝激酶B1/AMP活化蛋白激酶途径,阻止了甾醇调节元件结合蛋白-1向细胞核和叉头盒O1向细胞质的易位。α-硫辛酸增加了脂肪三酰甘油脂肪酶的表达,降低了脂肪酸合酶的丰度。在体内和体外研究中,α-硫辛酸还通过去乙酰化酶1途径增加了核NF-E2相关因子2水平和下游靶量。α-硫辛酸最终降低了肝内和血清甘油三酯含量。α-硫辛酸对肝脂肪变性的保护作用似乎与转录因子固醇调节元件结合蛋白-1、叉头盒O1和NF-E2相关因子2[3]有关。 |
| 细胞实验 |
人肝细胞癌 (HepG2) 细胞系在 37°C、5% CO2 和 10% 胎牛血清的 Dulbecco 改良 Eagle 培养基中生长。将以下物质应用于 HepG2 细胞:AMPK 抑制剂(CC,20 μM,0.5 小时)、SIRT1 抑制剂(NA,10 mM,12 或 24 小时)、AMPK 激活剂(AICAR,2 mM,1 小时)、棕榈酸酯(PA) ,125 μM,12 小时)和硫辛酸(250 μM,6 或 12 小时)[1]。
细胞系[4] 大鼠肝癌细胞系FaO和肝癌细胞系HepG2分别在Dulbecco培养基(DMEM加Glutamax I)中维持,并在5%CO2/95%空气的加湿气氛中补充青霉素、链霉素和10%热灭活胎牛血清(FCS),温度为37℃ °C.α-硫辛酸(α-LA)和Thapsigargin(TG)购自xxx。α-LA,溶于氢氧化钠NaOH 1 将N和在培养基中中和的TG以及溶解在DMSO中的TG加入到培养基中,达到文中规定的最终浓度。 细胞凋亡的形态学评估[4] 使用Hoechst 33258染色进行形态学评估和凋亡细胞检测。FaO细胞(2× 105 细胞/孔)被放置在室载玻片中,并在α-LA/硫辛酸存在或不存在的情况下进行培养。处理后,用2%多聚甲醛固定细胞,并用Hoescht 33258染色。在Leica DM2000荧光显微镜下观察染色细胞,并用Leica DCF420C数码相机获取图像。 |
| 动物实验 |
Mice: Male C57BL/6J mice (6 weeks old; body weight: 22-24 g) are divided into four groups (n=8) and given access to a normal diet and water ad libitum for two weeks. These groups are: normal diet (ND) (10% energy from fat), high-fat diet (HFD) (60% energy from fat), and HFD plus α-Lipoic Acid (100 mg/kg or 200 mg/kg). After the mice's eyes are removed for the preparation of the serum, blood samples are taken 24 weeks after the start of treatment. Centrifugation at 2000×g for 10 min. at 4 °C is used to separate the serum. Harvested in liquid nitrogen and kept at -80°C are the liver tissues.
Male C57BL/6J mice (6-week-old; body weight: 22–24 g) were housed in standard cage conditions at a constant temperature (22±1°C) and a 13:11-h light/dark cycle. All mice were allowed ad libitum access to normal diet and water for 2 weeks before dividing into four groups (n=8): normal diet (ND) (10% energy from fat; D12450B), high-fat diet (HFD) (60% energy from fat) and HFD plus ALA (100 mg/kg or 200 mg/kg). These doses of ALA/lipoic acid were selected to be similar to previous studies. After 24 weeks of treatment, blood samples were collected after the eyeballs of the mice were extracted for serum preparation by centrifugation at 2000×g for 10 min at 4°C. The liver tissues were harvested in liquid nitrogen and stored at −80°C. [3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
To determine the concentration of alpha-lipoic acid in the aqueous humour and investigate if its topical instillation can increase quantities. Methods: Seventy patients selected to undergo cataract surgery were randomly divided into two groups. Group 1 was used as a control group; for the patients in Group 2, a single instillation of alpha-lipoic acid eye drops (1%) was administered. Immediately before surgery an aliquot of 40-120 microL of aqueous humour was aspirated. The individual aspirations were combined to constitute pools representing time intervals with respect to administration. The levels of alpha-lipoic acid in the aqueous humour were measured using gas chromatography/mass-spectrometry. Pool 0 was created by combining the samples of aqueous humour obtained from the patients in Group 1, the control group, and the level of alpha-lipoic acid was 27.5 + 2.6 ng/mL; in the other pools the time interval between the administration of the eye drops and sampling was respectively 23 minutes, 53 minutes, 72 minutes, 93 minutes and 114 minutes, and the level of alpha-lipoic acid was 33.0 + 10.8 ng/mL; 52.0 + 2.5 ng/mL; 86.7 + 2.5 ng/mL; 91.2 + 2.5 ng/mL; 80.3 + 2.5 ng/mL. /The/ study demonstrates the presence of alpha-lipoic acid in the aqueous humour and indicates that its concentration increases after it is administered in the form of eye drops, reaching maximum values after around 93 minutes. The concentrations that are achieved in the anterior chamber allow us to theorise the possibility of exploiting the antioxidant properties of alpha-lipoic acid. R(+)-alpha-lipoic acid is a natural occurring compound that acts as an essential cofactor for certain dehydrogenase complexes. The redox couple alpha-lipoic acid/dihydrolipoic acid possesses potent antioxidant activity. Exogenous racemic alpha-lipoic acid orally administered for the symptomatic treatment of diabetic polyneuropathy is readily and nearly completely absorbed, with a limited absolute bioavailability of about 30% caused by high hepatic extraction. Although the pharmacokinetics of the parent drug have been well characterized in humans, relatively little is known regarding the excretion of alpha-lipoic acid and the pharmacokinetics of any metabolites in humans. In the present study, plasma concentration-time courses, urinary excreted amounts, and pharmacokinetic parameters of alpha-lipoic acid metabolites were evaluated in 9 healthy volunteers after multiple once-daily oral administration of 600 mg racemic alpha-lipoic acid. The primary metabolic pathways of alpha-lipoic acid in man, S-methylation and beta-oxidation, were quantitatively confirmed by an HPLC-electrochemical assay newly established prior to the beginning of this study. Major circulating metabolites were the S-methylated beta-oxidation products 4,6-bismethylthio-hexanoic acid and 2,4-bismethylthio-butanoic acid, whereas its conjugated forms accounted for the major portion excreted in urine. There was no statistically significant difference in the pharmacokinetic parameters Cmax, AUC, and tmax between day 1 and day 4. Despite the prolonged half-lives of the major metabolites compared to the parent drug, no evidence of accumulation was found. Mean values of 12.4% of the administered dose were recovered in the urine after 24 hours as the sum of alpha-lipoic acid and its metabolites. The results of the present study revealed that urinary excretion of alpha-lipoic acid and five of its main metabolites does not play a significant role in the elimination of alpha-lipoic acid. Therefore, biliary excretion, further electrochemically inactive degradation products, and complete utilization of alpha-lipoic acid as a primary substrate in the endogenous metabolism should be considered. In an open-label, parallel-group study involving 16 patients (8 with severely reduced renal function, 8 with end-stage renal disease needing hemodialysis), the effect of renal function on the pharmacokinetics, metabolism, and safety `of alpha-lipoic acid (thioctic acid) was evaluated by comparing the pharmacokinetic parameters with those of a reference group of 8 healthy subjects. Alpha-lipoic acid 600 mg was administered orally once daily for 4 days, and the pharmacokinetic parameters were measured on days 1 and 4. The mean percentage of the administered dose excreted in urine as parent compound was 0.2 and 0.05 in healthy subjects and subjects with severely reduced renal function, respectively. Assuming a bioavailability of 30%, this represents 0.67% and 0.17% of the bioavailable amount of alpha-lipoic acid, respectively. The percentage of total urinary recovered amounts of alpha-lipoic acid and 5 of its metabolites was 12.0 on both days. The respective values for patients with severe kidney damage were 5.2% (day 1) and 6.4% (day 4). The total percentage of the administered dose removed by hemodialysis was 4.0 in patients with end-stage renal disease. Renal clearance of alpha-lipoic acid and its major metabolites, 6,8-bismethylthio-octanoic acid, 4,6-bismethylthio-hexanoic acid and 2,4-bismethylthio-butanoic acid, were significantly decreased in subjects with kidney damage compared to the reference group. Apparent total clearance of alpha-lipoic acid was poorly correlated with creatinine clearance. There is strong evidence that alpha-lipoic acid is mainly excreted by nonrenal mechanism or further degraded to smaller units in the catabolic process. The significantly increased area under the curve values of 4,6-bismethylthio-hexanoic acid and half-lives of 2,4-bismethylthio-butanoic acid on both days in patients with severely reduced function and end-stage renal disease were not considered to be clinically relevant. Although trough levels of both metabolites tend to increase slightly in these subjects, no accumulation effects were detected. We conclude that the pharmacokinetics of alpha-lipoic acid are not influenced by creatinine clearance and are unaffected in subjects with severely reduced kidney function or end-stage renal disease. Hemodialysis did not significantly contribute to the clearance of alpha-lipoic acid. Hence, dose adjustment of alpha-lipoic acid is not necessary in patients with renal dysfunction. Alpha-lipoic aicd is absorbed from the small intestine and distributed to the liver via the portal circulation and to various tissues in the body via the systemic circulation.The natural R-enantiomer is more readily absorbed than the L-enantiomer and is the more active form. Alpha-lipoic acid readily crosses the blood-brain barrier. It is found, after its distribution to the various body tissues, intracellularly, intramitochondrialy and extracellularly. Metabolism / Metabolites Alpha-lipoic acid is metabolized to its reduced form, dihydrolipoic acid by mitochondrial lipoamide dehydrogenase. Dihydroipoic acid, together with lipoic acid, form a redox couple. It is also metabolized to lipoamide, which functions as the lipoic acid cofactor in the multienzyme complexes that catalyze the oxidative decarboxylations of pyruvate and alpha-ketoglutarate. Alpha-lipoic acid may be metabolized to dithiol octanoic acid, which can undergo catabolism. The excretion and biotransformation of rac-alpha-lipoic acid (LA), which is used for the symptomatic treatment of diabetic polyneuropathy, were investigated following single oral dosing of [(14)C]LA to mice (30 mg/kg), rats (30 mg/kg), dogs (10 mg/kg), and unlabeled LA to humans (600 mg). More than 80% of the radioactivity given was renally excreted. Metabolite profiles obtained by radiometric high-performance liquid chromatography revealed that LA was extensively metabolized irrespective of the species. Based on a new on-line liquid chromatography/tandem mass spectroscopy assay developed for negative ions, LA and a total of 12 metabolites were identified. Mitochondrial beta-oxidation played the paramount role in the metabolism of LA. Simultaneously, the circulating metabolites were subjected to reduction of the 1,2-dithiolane ring and subsequent S-methylation. In addition, evidence is given for the first time that the methyl sulfides formed were partly oxidized to give sulfoxides, predominantly in dogs. The disulfoxide of 2,4-bismethylmercapto-butanoic acid, the most polar metabolite identified, was the major metabolite in dogs. Furthermore, new data are presented that suggest conjugation with glycine occurred as a separate metabolic pathway in competition with beta-oxidation, predominantly in mice. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
ALA is generally considered a safe drug. A daily dose of 200 mg/day to 2400 mg/day of ALA is deemed safe without significant adverse effects. However, there is no reported safe dose in children. A notable case in literature demonstrated status epilepticus (SE) that subsided within a few days. The seizures were treated per normal standards for SE. In the last 2 decades, there have been few reported cases of ALA toxicity in humans. Most of these cases occur in children and are treatable. Though there is no established lethal dosage of ALA for humans, studies have shown that a high dose of 121 mg/kg body weight/day was associated with alterations in liver enzymes and liver function. Therefore, there are potentially harmful adverse effects from overdosing on ALA, and more studies are necessary to determine the toxicity. (R)-lipoic acid is a cholinesterase or acetylcholinesterase (AChE) inhibitor. A cholinesterase inhibitor (or 'anticholinesterase') suppresses the action of acetylcholinesterase. Because of its essential function, chemicals that interfere with the action of acetylcholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death. Nerve gases and many substances used in insecticides have been shown to act by binding a serine in the active site of acetylcholine esterase, inhibiting the enzyme completely. Acetylcholine esterase breaks down the neurotransmitter acetylcholine, which is released at nerve and muscle junctions, in order to allow the muscle or organ to relax. The result of acetylcholine esterase inhibition is that acetylcholine builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop. Among the most common acetylcholinesterase inhibitors are phosphorus-based compounds, which are designed to bind to the active site of the enzyme. The structural requirements are a phosphorus atom bearing two lipophilic groups, a leaving group (such as a halide or thiocyanate), and a terminal oxygen. Health Effects Acute exposure to cholinesterase inhibitors can cause a cholinergic crisis characterized by severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Accumulation of ACh at motor nerves causes overstimulation of nicotinic expression at the neuromuscular junction. When this occurs symptoms such as muscle weakness, fatigue, muscle cramps, fasciculation, and paralysis can be seen. When there is an accumulation of ACh at autonomic ganglia this causes overstimulation of nicotinic expression in the sympathetic system. Symptoms associated with this are hypertension, and hypoglycemia. Overstimulation of nicotinic acetylcholine receptors in the central nervous system, due to accumulation of ACh, results in anxiety, headache, convulsions, ataxia, depression of respiration and circulation, tremor, general weakness, and potentially coma. When there is expression of muscarinic overstimulation due to excess acetylcholine at muscarinic acetylcholine receptors symptoms of visual disturbances, tightness in chest, wheezing due to bronchoconstriction, increased bronchial secretions, increased salivation, lacrimation, sweating, peristalsis, and urination can occur. Certain reproductive effects in fertility, growth, and development for males and females have been linked specifically to organophosphate pesticide exposure. Most of the research on reproductive effects has been conducted on farmers working with pesticides and insecticdes in rural areas. In females menstrual cycle disturbances, longer pregnancies, spontaneous abortions, stillbirths, and some developmental effects in offspring have been linked to organophosphate pesticide exposure. Prenatal exposure has been linked to impaired fetal growth and development. Neurotoxic effects have also been linked to poisoning with OP pesticides causing four neurotoxic effects in humans: cholinergic syndrome, intermediate syndrome, organophosphate-induced delayed polyneuropathy (OPIDP), and chronic organophosphate-induced neuropsychiatric disorder (COPIND). These syndromes result after acute and chronic exposure to OP pesticides. Symptoms Symptoms of low dose exposure include excessive salivation and eye-watering. Acute dose symptoms include severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Hypertension, hypoglycemia, anxiety, headache, tremor and ataxia may also result. Treatment If the compound has been ingested, rapid gastric lavage should be performed using 5% sodium bicarbonate. For skin contact, the skin should be washed with soap and water. If the compound has entered the eyes, they should be washed with large quantities of isotonic saline or water. In serious cases, atropine and/or pralidoxime should be administered. Anti-cholinergic drugs work to counteract the effects of excess acetylcholine and reactivate AChE. Atropine can be used as an antidote in conjunction with pralidoxime or other pyridinium oximes (such as trimedoxime or obidoxime), though the use of '-oximes' has been found to be of no benefit, or possibly harmful, in at least two meta-analyses. Atropine is a muscarinic antagonist, and thus blocks the action of acetylcholine peripherally. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
/EXPERIMENTAL THERAPY/ The aim of this trial was to evaluate the effects of alpha-lipoic acid (ALA) on positive sensory symptoms and neuropathic deficits in diabetic patients with distal symmetric polyneuropathy (DSP). In this multicenter, randomized, double-blind, placebo-controlled trial, 181 diabetic patients in Russia and Israel received once-daily oral doses of 600 mg (n = 45) (ALA600), 1,200 mg (n = 47) (ALA1200), and 1,800 mg (ALA1800) of ALA (n = 46) or placebo (n = 43) for 5 weeks after a 1-week placebo run-in period. The primary outcome measure was the change from baseline of the Total Symptom Score (TSS), including stabbing pain, burning pain, paresthesia, and asleep numbness of the feet. Secondary end points included individual symptoms of TSS, Neuropathy Symptoms and Change (NSC) score, Neuropathy Impairment Score (NIS), and patients' global assessment of efficacy. Mean TSS did not differ significantly at baseline among the treatment groups and on average decreased by 4.9 points (51%) in ALA600, 4.5 (48%) in ALA1200, and 4.7 (52%) in ALA1800 compared with 2.9 points (32%) in the placebo group (all P < 0.05 vs. placebo). The corresponding response rates (> or = 50% reduction in TSS) were 62, 50, 56, and 26%, respectively. Significant improvements favoring all three ALA groups were also noted for stabbing and burning pain, the NSC score, and the patients' global assessment of efficacy. The NIS was numerically reduced. Safety analysis showed a dose-dependent increase in nausea, vomiting, and vertigo. CONCLUSIONS: Oral treatment with ALA for 5 weeks improved neuropathic symptoms and deficits in patients with DSP. An oral dose of 600 mg once daily appears to provide the optimum risk-to-benefit ratio. /EXPERIMENTAL THERAPY/ Mitochondria produce reactive oxygen species that may contribute to vascular dysfunction. alpha-Lipoic acid and acetyl-L-carnitine reduce oxidative stress and improve mitochondrial function. In a double-blind crossover study, the authors examined the effects of combined alpha-lipoic acid/acetyl-L-carnitine treatment and placebo (8 weeks per treatment) on vasodilator function and blood pressure in 36 subjects with coronary artery disease. Active treatment increased brachial artery diameter by 2.3% (P=.008), consistent with reduced arterial tone. Active treatment tended to decrease systolic blood pressure for the whole group (P=.07) and had a significant effect in the subgroup with blood pressure above the median (151+/-20 to 142+/-18 mm Hg; P=.03) and in the subgroup with the metabolic syndrome (139+/-21 to 130+/-18 mm Hg; P=.03). Thus, mitochondrial dysfunction may contribute to the regulation of blood pressure and vascular tone.... /EXPERIMENTAL THERAPY/ Lipoic acid is an antioxidant that suppresses and treats an animal model of multiple sclerosis, experimental autoimmune encephalomyelitis. The purpose of this study was to determine the pharmacokinetics (PK), tolerability and effects on matrix metalloproteinase-9 (MMP-9) and soluble intercellular adhesion molecule-1 (sICAMP-1) of oral lipoic acid in patients with multiple sclerosis. Thirty-seven MS subjects were randomly assigned to one of four groups: placebo, lipoic acid 600 mg twice a day, lipoic acid 1200 mg once a day and lipoic acid 1200 mg twice a day. Subjects took study capsules for 14 days. ... Subjects taking 1200 mg lipoic acid had substantially higher peak serum lipoic acid levels than those taking 600 mg and that peak levels varied considerably among subjects. We also found a significant negative correlation between peak serum lipoic acid levels and mean changes in serum MMP-9 levels (T = -0.263, P =0.04). There was a significant dose response relationship between lipoic acid and mean change in serum sICAM-1 levels (P =0.03). ... Oral lipoic acid is generally well tolerated and appears capable of reducing serum MMP-9 and sICAM-1 levels. Lipoic acid may prove useful in treating MS by inhibiting MMP-9 activity and interfering with T-cell migration into the CNS. /EXPERIMENTAL THERAPY/ Mitochondrial dysfunction and oxidative damage are highly involved in the pathogenesis of Parkinson's disease. Some mitochondrial antioxidants/nutrients that can improve mitochondrial function and/or attenuate oxidative damage have been implicated in Parkinson's disease therapy. However, few studies have evaluated the preventative effects of a combination of mitochondrial antioxidants/nutrients against Parkinson's disease, and even fewer have sought to optimize the doses of the combined agents. The present study examined the preventative effects of two mitochondrial antioxidant/nutrients, R-alpha-lipoic acid (LA) and acetyl-L-carnitine (ALC), in a chronic rotenone-induced cellular model of Parkinson's disease. We demonstrated that 4-week pretreatment with LA and/or ALC effectively protected SK-N-MC human neuroblastoma cells against rotenone-induced mitochondrial dysfunction, oxidative damage, and accumulation of alpha-synuclein and ubiquitin. Most notably, we found that when combined, LA and ALC worked at 100 to 1000 fold lower concentrations than they did individually. We also found that pretreatment with combined LA and ALC increased mitochondrial biogenesis and decreased production of reactive oxygen species through the upregulation of the peroxisome proliferator-activated receptor-gamma coactivator 1alpha as a possible underlying mechanism. This study provides important evidence that combining mitochondrial antioxidant/nutrients at optimal doses might be an effective and safe prevention strategy for Parkinson's disease. /R-alpha-lipoic acid/ For more Therapeutic Uses (Complete) data for alpha-Lipoic acid (11 total), please visit the HSDB record page. Drug Warnings Those with diabetes and problems with glucose intolerance are cautioned that supplemental alpha-lipoic acid may lower blood glucose levels. Blood glucose should be monitored and antidiabetic drug dose adjusted, if necessary, to avoid possible hypoglycemia. Because of lack of long-term safety data, alpha-lipoic acid should be avoided by pregnant and nursing mothers. The "Long Terminal Repeat" (LTR) of HIV-1 is the target of cellular transcription factors such as NF-kappaB, and serves as the promoter-enhancer for the viral genome when integrated in host DNA. Various LTR-reporter gene constructs have been used for in vitro studies of activators or inhibitors of HIV-1 transcription, e.g., to show that antioxidants such as lipoic acid and selenium inhibit NF-kappaB-dependent HIV-1 LTR activation. One such construct is the pHIVlacZ plasmid, with the HIV-1 LTR driving expression of the lacZ gene (encoding beta-galactosidase, beta-gal). Typically, for inhibitor screening, cells transfected with pHIVlacZ are activated using tumor necrosis factor-alpha (TNF-alpha), and the colorimetric o-nitrophenol assay is used to assess changes in beta-gal activity. A variant of this assay was developed as described here, in which LTR activation was induced by pro-fs, a novel HIV-1 gene product encoded via a -1 frameshift from the protease gene. Cotransfection of cells with pHIVlacZ along with a pro-fs construct produced a significant increase in beta-gal activity over controls. L-ergothioneine dose dependently inhibited both TNF-alpha-mediated and pro-fs-mediated increases in beta-gal activity, with an IC50 of about 6 mM. Thus antioxidant strategy involving ergothioneine derived from food plants might be of benefit in chronic immunodeficiency diseases.[1] Retinal ischemia-reperfusion (RIR) injury causes neuronal degeneration and initiates various optic nerve diseases. This study aimed to investigate the synergistic neuroprotective effect of rasagiline and idebenone against RIR injury. A combination of rasagiline and idebenone was administered intraperitoneally immediately after establishment of the RIR model. Treatment with the combination of the two drugs resulted in a significant restoration of retinal thickness and retinal ganglion cells. Apoptosis of cells in ganglion cell layers was also ameliorated, suggesting that the effect of the two drugs was synergistic and the expression of brain-derived neurotrophic factor increased. Furthermore, idebenone and rasagiline induced the expression of Lin28A and Lin28B, respectively, which resulted in a reduced expression of microRNAs in the let-7 family and an increased protein output of Dicer. The data obtained from gene overexpression and knockdown experiments indicated that let-7 and Dicer were necessary for the synergistic neuroprotective effect of the two drugs. Our findings suggested that combination therapy with rasagiline and idebenone produced a synergistic effect that ameliorated RIR injury and restored visual function. In addition, the combined treatment provided neuroprotection via enhancement of the selective regulation of let-7 by Lin28A/B. These findings implied that a treatment with the combination of rasagiline and idebenone is a feasible treatment option for optic nerve diseases.[2] |
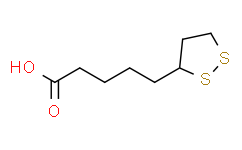
| 分子式 |
C₈H₁₄O₂S₂
|
|---|---|
| 分子量 |
206.3256
|
| 精确质量 |
206.043
|
| 元素分析 |
C, 46.57; H, 6.84; O, 15.51; S, 31.08
|
| CAS号 |
1077-28-7
|
| 相关CAS号 |
α-Lipoic Acid;1077-28-7
|
| PubChem CID |
864
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
362.5±11.0 °C at 760 mmHg
|
| 熔点 |
60-62ºC
|
| 闪点 |
173.0±19.3 °C
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
| 折射率 |
1.562
|
| LogP |
2.16
|
| tPSA |
87.9
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
12
|
| 分子复杂度/Complexity |
150
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S1C([H])(C([H])([H])C([H])([H])S1)C([H])([H])C([H])([H])C([H])([H])C([H])([H])C(=O)O[H]
|
| InChi Key |
AGBQKNBQESQNJD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C8H14O2S2/c9-8(10)4-2-1-3-7-5-6-11-12-7/h7H,1-6H2,(H,9,10)
|
| 化学名 |
5-(dithiolan-3-yl)pentanoic acid
|
| 别名 |
Lipoic Acid; (R)-5-(1,2-Dithiolan-3-yl)pentanoic acid; R-(+)-alpha-Lipoic acid; (+)-alpha-Lipoic acid; Verla-Lipon; Lipoate; Verla Lipon; VerlaLipon; Thioctic Acid; Thioctacide T; Thiogamma Injekt; Thiogamma oral; thioctic acid; dl-Thioctic acid; 1077-28-7; alpha-Lipoic acid; lipoic acid; 5-(1,2-Dithiolan-3-yl)pentanoic acid; DL-alpha-Lipoic acid; 1,2-dithiolane-3-pentanoic acid;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~484.7 mM)
H2O: < 0.1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.8466 mL | 24.2330 mL | 48.4660 mL | |
| 5 mM | 0.9693 mL | 4.8466 mL | 9.6932 mL | |
| 10 mM | 0.4847 mL | 2.4233 mL | 4.8466 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03161028 | Active Recruiting |
Drug: Lipoic acid Drug: Placebo |
Multiple Sclerosis | VA Office of Research and Development |
July 1, 2018 | Phase 2 |
| NCT00765310 | Active Recruiting |
Dietary Supplement: R-alpha lipoic acid Dietary Supplement: Placebo |
Atherosclerosis | Oregon State University | April 2009 | Phase 2 Phase 3 |
| NCT00764270 | Active Recruiting |
Dietary Supplement: R-alpha lipoic acid |
Atherosclerosis | Oregon State University | August 2011 | Phase 2 Phase 3 |
| NCT02910531 | Active Recruiting |
Dietary Supplement: Alpha lipoic acid Drug: Placebo |
Cystinuria | Thomas Chi, MD | June 19, 2017 | Phase 2 |
| NCT02168140 | Active Recruiting |
Drug: bendamustine hydrochloride Drug: 6,8-bis(benzylthio)octanoic acid |
Peripheral T-cell Lymphoma Hepatosplenic T-cell Lymphoma |
Wake Forest University Health Sciences |
September 2014 | Phase 1 |