| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |

| 靶点 |
EC50: 34 pM (GT1a), 4 pM (GT1b)[1] IC50: 1.62 Μm (SARS-CoV 3CLpro)[3]
|
|---|---|
| 体外研究 (In Vitro) |
盐酸雷迪帕韦的内在 EC50 值为 39,对于 GT1a 为 310 fM,对于 GT1b 为 40 fM。 GT1a 的蛋白质调整 EC50 值为 210 pM,GT1b 的蛋白质调整 EC50 值为 27 pM。 GT1a 和 1b EC50 值分别为 31 pM 和 4 pM。复制子实验中使用的人血清和细胞培养基均含有 10% BSA,盐酸雷迪帕韦的蛋白结合水平显着[1]。当用于对抗 JFH/3a-NS5A 复制子时,盐酸雷迪帕韦的 EC50 值为 141 nM [2]。
|
| 体内研究 (In Vivo) |
盐酸雷迪帕韦的独特之处在于其低清除率、良好的生物利用度、在大鼠、狗和猴子中的半衰期长,以及在人类中的低预计清除率以及高复制子效力。在大鼠和狗中评估了盐酸雷迪帕韦的药代动力学。 Ledipasvir 表现出较低的全身清除率 (CL)、中等分布容积 (Vss)(大于全身水量)以及良好的血浆半衰期(大鼠 1.83 小时,狗 2.63 小时)[1]。
|
| 酶活实验 |
竞争性蛋白结合试验[1]
将含有10%胎牛血清(CCM)的人血浆和细胞培养基以2μM的终浓度掺入受试化合物。将加标血浆(1 mL)和CCM(1 mL)放入组装好的透析细胞的相对侧,用半透膜隔开。透析细胞在37°C水浴中缓慢旋转达到平衡所需的时间。测量透析后血浆和CCM重量,并用LC/MS/MS测定血浆和CCM中的试验化合物浓度。 代谢稳定性[1] 使用合并的肝微粒体组分(最终蛋白质浓度为0.5mg/mL)在3μM的最终试验化合物浓度下测定体外代谢稳定性。通过加入NADPH再生系统引发反应。在不同时间点将25μL的反应混合物等分转移到含有淬火溶液的板上。反应混合物中的试验化合物浓度用LC/MS/MS测定。肝脏固有清除率如Obach之前所述计算,预测清除率使用搅拌良好的肝脏模型计算,不受蛋白质限制。 还使用氚化测试化合物在冷冻保存的肝细胞中测定了代谢稳定性。孵育混合物含有1×106个肝细胞/mL和1μM氚化试验化合物(2.5μCi)。在37°C的温度下,在95%空气/5%二氧化碳(v/v)的潮湿环境中轻轻摇晃进行孵化。在0、1、3和6小时后取出50μL的等分试样,并将其加入100μL的淬火溶液中。在与HPLC系统耦合的流动闪烁无线电探测器上分析样品。代谢物根据放射性检测器的峰面积进行定量,无细胞对照样品用作参考。通过测量测试化合物的消失率,即形成的放射性标记代谢物和测试化合物的总峰面积的百分比,来确定肝细胞中的代谢稳定性。 |
| 细胞实验 |
GT1a and GT1b Replicons[1]
稳定的基因型1a(GT1a)亚基因组复制子细胞系1a-57C-RlucP(H77菌株)用于测定化合物GT1a的抗病毒活性,并如前所述建立。在稳定的GT1b亚基因组复制子细胞系1b-Luc-2(Con-1菌株)中测定了化合物GT1b的抗病毒活性。为了建立1b-Luc-2,从ReBLikon获得的质粒I389luc-ubineo/NS3-3′/ET中产生了复制子质粒pCon1/SG-hRlucNeo(G+I+T),该质粒编码Con-1菌株的亚基因组复制子。使用Accuprime Super Mix I和引物AscI-hRluc-Fwd和NotI-hRluc Rev通过PCR从pF9 CMV hRluc-Neo Flexi中扩增出hRluc-Neo基因。这两个引物具有以下序列,并携带限制性位点以供后续克隆:AscI-hRuc-Fwd:5′-ACT GAC GGC GCG CCA TGG CTT CCA AGG TGT ACG-3′(AscI位点下划线)和NotI-hMluc Rev:5′-GTC AGT GCG GCT CAG AAG AAC TCG TCA AGA-3′(NotI位点划线)。将hRluc-Neo扩增产物亚克隆到pCR2.1-TOPO中。用AscI和NotI消化所得质粒,用T4 DNA连接酶将切下的片段(hRluc-Neo)连接到用相同酶消化的I389luc-ubi-Neo/NS3-3′/ET中。对所得载体pCon1/SG-hRlucNeo(G+I+T)进行测序,以确认hRluc-Neo融合基因的正确方向和序列。 质粒pCon1/SG-hRlucNeo(G+I+T)用SpeI线性化,并使用PCR纯化试剂盒纯化。按照制造商建议的方案,用T7MEGAScript试剂体外合成复制子RNA。根据制造商的说明,使用RNeasy试剂盒通过柱纯化纯化RNA。通过测量260nm处的吸光度来确定RNA浓度,并通过0.8%琼脂糖凝胶电泳和溴化乙锭染色来验证其完整性。如前所述,将10微克体外转录的pCon1/SG-hRlucNeo(G+I+T)RNA电穿孔到4×106 Huh7-Lunet细胞中。简而言之,将电穿孔细胞铺在100mm细胞培养皿上。镀覆24小时后,用补充了1.0 mg/mL G418的繁殖培养基替换培养基(选择持续约3周)。分离并扩增G418抗性克隆。根据制造商的说明,使用商业化的Renilla萤光素酶测定法对HCV复制进行定量。选择具有最高荧光素酶信号与背景比的克隆在高通量抗病毒敏感性试验中进行验证。用于GT1b抗病毒研究的最终克隆细胞系被命名为1b-Luc-2。 Replicon抗病毒检测[1] 为了确定化合物GT1的抗病毒活性,将1a-57C-RlucP或1b-Luc-2复制子细胞以每孔2000个细胞的速度铺在384孔板上(细胞培养处理)。将化合物在DMSO中连续稀释3倍,并使用自动仪器以0.44%DMSO的终浓度加入细胞中,总体积为90μL。对于每种药物浓度,在384孔板上设置四孔。DMSO用作阴性(溶剂;无抑制)对照,三种HCV抑制剂的组合,包括蛋白酶抑制剂、NS5A抑制剂和核苷抑制剂,以>100×EC50的浓度用作阳性对照(100%抑制)。将板在37°C、5%CO2和85%湿度的环境中孵育3天。用Biotek ELX405洗板机吸出培养基。使用BiotekμFlow Workstation将20微升双Glo萤光素酶缓冲液添加到平板的每个孔中。将平板在室温下孵育10分钟。使用BiotekμFlow Workstation向每个孔中加入20微升含有双Glo Stop&Glo底物和双Glo Stop&Glo缓冲液的1:100混合物的溶液。将平板在室温下孵育10分钟,然后用Envision平板读数器测量发光信号。 |
| 动物实验 |
Mice: Recombinant adenovirus Ad-WT-HCVpro-SEAP, with 109 IFU per mouse, is injected via the tail vein into five groups of six-week-old SCID mice (six animals per group). Two oral doses of Telaprevir (VX-950) at a dose of 10, 25, 75, 150, or 300 mg/kg are administered to each group of mice. First dose of Telaprevir is administered two hours prior to adenovirus injection; second dose is administered ten hours following injection. A second set of ten mice is given the vehicle on its own. Serum samples are taken 24 hours after injection, and the SEAP activity in each group administered with Telaprevir is contrasted with the vehicle group's. Rat and Canine Rats and dogs are used to assess the oral and intravenous pharmacokinetics of telaprevir (VX-950). One intravenous bolus dose of 0.95 mg/kg Telaprevir is given intravenously to three male Sprague-Dawley rats weighing 250–300 g. Heparinized tubes are used to collect serial blood samples prior to dosage administration and at intervals of 0.083, 0.167, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, and 8 hours following the dose. Telaprevir in 10% ethanol, 40% polyethylene glycol 400, and 50% D5W is given intravenously as a bolus dose to three male beagle dogs (8–12 kg). Heparinized tubes are used to collect serial blood samples prior to dosage administration as well as at 0.083, 0.167, 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, 12, and 24 hours later. Telaprevir is formulated in polyvinylpyrrolidone (PVP) K-30 plus 2% sodium lauryl sulfate and dosed as an oral gavage for oral studies in rats and dogs. Oral dosages of 40 mg/kg VX-950 are given to three male Sprague-Dawley rats (250–300 g) and 9.6 mg/kg VX-950 are given to four male Beagle dogs (10.9–12.0 kg). Blood samples are obtained before dosage administration and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 hours following dose administration in both oral studies. Plasma samples are obtained by centrifugation and kept at -70°C until analysis in both intravenous and oral studies. Samples from the oral studies are analyzed using an achiral LC/MS/MS method, while samples from the intravenous studies are analyzed using a chiral liquid chromatography followed by tandem mass spectrometry (LC/MS/MS) method.
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption When given orally, ledipasvir reaches its maximum plasma concentration in about 4 to 4.5 hours with a maximum concentration (Cmax) of 323 ng/mL. Route of Elimination Following a single 90 mg oral dose of [14C]-ledipasvir, mean total recovery of the [14C]-radioactivity in feces and urine was approximately 87%, with most of the radioactive dose recovered from feces (approximately 86%). Unchanged ledipasvir excreted in feces accounted for a mean of 70% of the administered dose and the oxidative metabolite M19 accounted for 2.2% of the dose. These data indicate that biliary excretion of unchanged ledipasvir is a major route of elimination, with renal excretion being a minor pathway (approximately 1%). Metabolism / Metabolites In vitro, no detectable metabolism of ledipasvir was observed by human CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Evidence of slow oxidative metabolism via an unknown mechanism has been observed. Following a single dose of 90 mg [14C]-ledipasvir, systemic exposure was almost exclusively to the parent drug (>98%). Unchanged ledipasvir is the major species present in feces. Biological Half-Life The median terminal half-life of ledipasvir is 47 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Ledipasvir has not been studied in nursing mothers being treated for hepatitis C infection. Because it is 99.8% bound to maternal plasma proteins, amounts in breastmilk are likely to be very low. If ledipasvir alone or in combination with sofosbuvir (Harvoni) is required by the mother, it is not a reason to discontinue breastfeeding. Some sources recommend against breastfeeding when ledipasvir is used with ribavirin. Hepatitis C is not transmitted through breastmilk and breastmilk has been shown to inactivate hepatitis C virus (HCV). However, the Centers for Disease Control recommends that mothers with HCV infection should consider abstaining from breastfeeding if their nipples are cracked or bleeding. It is not clear if this warning would apply to mothers who are being treated for hepatitis C. Infants born to mothers with HCV infection should be tested for HCV infection; because maternal antibody is present for the first 18 months of life and before the infant mounts an immunologic response, nucleic acid testing is recommended. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Drugs and Lactation Database (LactMed) Protein Binding Ledipasvir is >99.8% bound to human plasma proteins. |
| 参考文献 |
|
| 其他信息 |
Ledipasvir is a direct acting antiviral (DAA) medication used as part of combination therapy to treat chronic Hepatitis C, an infectious liver disease caused by infection with Hepatitis C Virus (HCV). HCV is a single-stranded RNA virus that is categorized into nine distinct genotypes, with genotype 1 being the most common in the United States, and affecting 72% of all chronic HCV patients. Treatment options for chronic Hepatitis C have advanced significantly since 2011, with the development of Direct Acting Antivirals (DAAs) such as ledipasvir. More specifically, ledipasvir is an inhibitor of the Hepatitis C Virus (HCV) Non-Structural Protein 5A (NS5A), which is required for viral RNA replication and assembly of HCV virions. Although its exact mechanism of action is unknown, it is postulated to prevent hyperphosphorylation of NS5A which is required for viral protein production. It is effective against genotypes 1a, 1b, 4a, and 5a and with a lesser activity against genotypes 2a and 3a of HCV. Ledipasvir and other direct acting antivirals are very potent options for the treatment of Hepatitis C, as they exhibit a high barrier to the development of resistance. This is an important advantage relative to HCV drugs that target other viral enzymes such as the protease, for which rapid development of resistance has proven to be an important cause of therapeutic failure. In a joint recommendation published in 2016, the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) recommend ledipasvir as a first line therapy option in combination with [sofosbuvir] for the treatment of HCV genotypes 1a, 1b, 4, 5, and 6. Treatment with ledipasvir is used with the intent to cure, or achieve a sustained virologic response (SVR), after 12 weeks of daily therapy. SVR and eradication of HCV infection is associated with significant long-term health benefits including reduced liver-related damage, improved quality of life, reduced incidence of Hepatocellular Carcinoma, and reduced all-cause mortality. Treatment with direct acting antivirals such as ledipasvir is associated with very minimal side effects, with the most common being headache and fatigue. Lack of significant side effects and short duration of therapy is a considerable advantage over older interferon- and ribavirin-based regimens, which were limited by infusion site reactions, reduced blood count, and neuropsychiatric effects. Since 2014, ledipasvir has been available as a fixed dose combination product with [sofosbuvir] (tradename Harvoni) used for the treatment of chronic Hepatitis C. Approved in October 2014 by the FDA, Harvoni is indicated for the treatment of HCV genotypes 1, 4, 5, and 6 with or without [ribavirin] depending on the level of liver damage or cirrhosis. When combined together, ledipasvir and sofosbuvir as the combination product Harvoni has been shown to achieve a SVR between 93 and 99% after 12 weeks of treatment. Its use has also proven successful in the treatment of HCV in patients co-infected with HIV.
|
| 分子式 |
C49H55CLF2N8O6
|
|---|---|
| 分子量 |
925.46
|
| 精确质量 |
924.3901
|
| CAS号 |
2128695-48-5
|
| 相关CAS号 |
Ledipasvir;1256388-51-8;Ledipasvir (acetone);1441674-54-9;Ledipasvir D-tartrate;1502654-87-6;Ledipasvir-d6;2050041-12-6;Ledipasvir (diacetone);1502655-48-2
|
| PubChem CID |
164887328
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
175Ų
|
| InChi Key |
DDPFJRIMZUVTQU-NDANSHMASA-N
|
| InChi Code |
InChI=1S/C49H54F2N8O6.ClH/c1-24(2)39(56-46(62)64-5)44(60)58-23-48(15-16-48)21-38(58)42-52-22-37(55-42)28-9-13-32-31-12-8-26(18-33(31)49(50,51)34(32)19-28)27-10-14-35-36(20-27)54-43(53-35)41-29-7-11-30(17-29)59(41)45(61)40(25(3)4)57-47(63)65-6;/h8-10,12-14,18-20,22,24-25,29-30,38-41H,7,11,15-17,21,23H2,1-6H3,(H,52,55)(H,53,54)(H,56,62)(H,57,63);1H/t29-,30+,38-,39-,40-,41-;/m0./s1
|
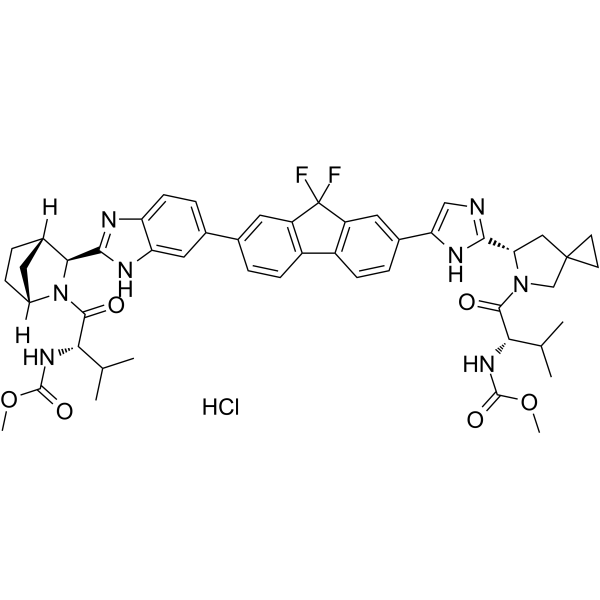
| 化学名 |
methyl N-[(2S)-1-[(6S)-6-[5-[9,9-difluoro-7-[2-[(1R,3S,4S)-2-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]-2-azabicyclo[2.2.1]heptan-3-yl]-3H-benzimidazol-5-yl]fluoren-2-yl]-1H-imidazol-2-yl]-5-azaspiro[2.4]heptan-5-yl]-3-methyl-1-oxobutan-2-yl]carbamate;hydrochloride
|
| 别名 |
Ledipasvir hydrochloride; Ledipasvir (hydrochloride);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.0805 mL | 5.4027 mL | 10.8054 mL | |
| 5 mM | 0.2161 mL | 1.0805 mL | 2.1611 mL | |
| 10 mM | 0.1081 mL | 0.5403 mL | 1.0805 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。