| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
H-ras (IC50 = 1.9 nM); K-ras (IC50 = 5.2 nM); N-ras (IC50 = 2.8 nM)[1]
|
|---|---|
| 体内研究 (In Vivo) |
我们一直在开发一系列非肽、小分子法尼烷基蛋白转移酶抑制剂,它们共享一个共同的三环核,并与肽/蛋白底物竞争与法尼烷基蛋白质转移酶的结合。在这里,我们报告了SCH 66336的药理学和体内研究,SCH 66336是这一结构类别中的先导化合物。SCH 66336能有效抑制整个细胞中的Ha-Ras加工,并阻断表达活化Ki-Ras蛋白的成纤维细胞和人肿瘤细胞系的转化生长特性。许多缺乏活化ras癌基因的人类肿瘤系的锚定非依赖性生长也被SCH 66336治疗阻断。在小鼠、大鼠和猴子系统中,SCH 66336具有优异的口服生物利用度和药代动力学特性。在裸小鼠中,SCH 66336在各种人类肿瘤异种移植物模型中表现出强大的口服活性,包括结肠、肺、胰腺、前列腺和膀胱来源的肿瘤。当SCH 66336与各种细胞毒性药物(环磷酰胺、5-氟尿嘧啶和长春新碱)联合使用时,观察到体内疗效增强。在Ha-Ras转基因小鼠模型中,SCH 66336的预防性治疗延迟了肿瘤发作,减少了每只小鼠的平均肿瘤数量,并降低了每只动物的平均肿瘤重量。在转基因小鼠出现可触及的肿瘤后开始灌胃治疗的治疗模式中,SCH 66336以剂量依赖的方式诱导了显著的肿瘤消退。这与用SCH 66336治疗的动物肿瘤中细胞凋亡增加和DNA合成减少有关。当SCH 66336与环磷酰胺联合使用时,在该模型中也观察到疗效增强。SCH 66336目前正在I期临床试验中进行评估[1]。
|
| 药代性质 (ADME/PK) |
Absorption
The absolute oral bioavailability of lonafarnib is unknown; in healthy subjects administration of either 75 or 100 mg of lonafarnib twice daily resulted in mean peak plasma concentrations (%CV) of 834 (32%) and 964 (32%) ng/mL, respectively. Twice daily administration of 115 mg/m2 lonafarnib in HGPS patients resulted in a median tmax of 2 hours (range 0-6), mean Cmax of 1777 ± 1083 ng/mL, mean AUC0-8hr of 9869 ± 6327 ng\*hr/mL, and a mean AUCtau of 12365 ± 9135 ng\*hr/mL. The corresponding values for a dose of 150 mg/m2 are: 4 hours (range 0-12), 2695 ± 1090 ng/mL, 16020 ± 4978 ng\*hr/mL, and 19539 ± 6434 ng\*hr/mL, respectively. Following a single oral dose of 75 mg in healthy subjects, the Cmax of lonafarnib decreased by 55% and 25%, and the AUC decreased by 29% and 21% for a high/low-fat meal compared to fasted conditions. Route of Elimination Up to 240 hours following oral administration of 104 mg [14C]-lonafarnib in fasted healthy subjects, approximately 62% and <1% of the initial radiolabeled dose was recovered in feces and urine, respectively. The two most prevalent metabolites were the active HM21 and HM17, which account for 14% and 15% of plasma radioactivity. Volume of Distribution In healthy patients administered either 75 or 100 mg lonafarnib twice daily, the steady-state apparent volumes of distribution were 97.4 L and 87.8 L, respectively. Metabolism / Metabolites Lonafarnib is metabolized _in vitro_ primarily by CYP3A4/5 and partially by CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, and CYP2E1. Formation of the primary metabolites involves oxidation and subsequent dehydration in the pendant piperidine ring. Biological Half-Life Lonafarnib has a mean half-life of approximately 4-6 hours following oral administration of 100 mg twice daily in healthy subjects. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the small prelicensure clinical trials conducted in children with progeria, serum aminotransferase elevations occurred in 35% of lonafarnib treated subjects but were usually mild and self-limited, rising to above 3 times the upper limit of normal (ULN) in only 5%. There were no liver related serious adverse events and no patient had a concurrent elevation in serum aminotransferase and bilirubin levels. Since approval of lonafarnib, there have been no published reports of drug induced liver injury associated with its use, although clinical experience with the drug, particularly with long term therapy, has been limited. Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury). Protein Binding Lonafarnib exhibits _in vitro_ plasma protein binding of ≥99% over a concentration range of 0.5-40.0 μg/mL. |
| 参考文献 | |
| 其他信息 |
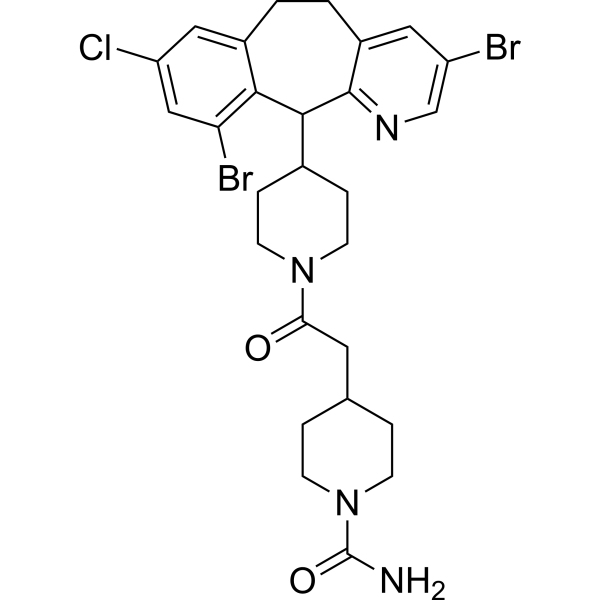
4-{2-[4-(3,10-dibromo-8-chloro-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-yl)piperidin-1-yl]-2-oxoethyl}piperidine-1-carboxamide is a benzocycloheptapyridine that is benzo[5,6]cyclohepta[1,2-b]pyridine which is substituted at positions 3 and 10 by bromines, at position 8 by chlorine, and at position 11 by an N-acetylpiperidin-4-yl group in which one of the hydrogens of the acetyl moiety has been replaced by a 1-carbamoylpiperidin-4-yl group. It is a benzocycloheptapyridine, a N-acylpiperidine, a heteroarylpiperidine, an organochlorine compound, an organobromine compound and a member of ureas.
|
| 分子式 |
C27H31BR2CLN4O2
|
|---|---|
| 分子量 |
638.821644067764
|
| 精确质量 |
636.05
|
| 元素分析 |
C, 50.76; H, 4.89; Br, 25.02; Cl, 5.55; N, 8.77; O, 5.01
|
| CAS号 |
193275-86-4
|
| 相关CAS号 |
Lonafarnib;193275-84-2
|
| PubChem CID |
9852353
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
710.4±70.0 °C at 760 mmHg
|
| 闪点 |
383.5±35.7 °C
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
| 折射率 |
1.630
|
| LogP |
5.03
|
| tPSA |
79.5
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
790
|
| 定义原子立体中心数目 |
0
|
| SMILES |
BrC1=CC(=CC2CCC3=CC(=CN=C3[C@@H](C=21)C1CCN(C(CC2CCN(C(N)=O)CC2)=O)CC1)Br)Cl
|
| InChi Key |
DHMTURDWPRKSOA-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C27H31Br2ClN4O2/c28-20-12-19-2-1-18-13-21(30)14-22(29)24(18)25(26(19)32-15-20)17-5-9-33(10-6-17)23(35)11-16-3-7-34(8-4-16)27(31)36/h12-17,25H,1-11H2,(H2,31,36)
|
| 化学名 |
4-[2-[4-(6,15-dibromo-13-chloro-4-azatricyclo[9.4.0.03,8]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-yl)piperidin-1-yl]-2-oxoethyl]piperidine-1-carboxamide
|
| 别名 |
Lonafarnib (Racemate); 193275-86-4; 4-{2-[4-(3,10-DIBROMO-8-CHLORO-6,11-DIHYDRO-5H-BENZO[5,6]CYCLOHEPTA[1,2-B]PYRIDIN-11-YL)PIPERIDIN-1-YL]-2-OXOETHYL}PIPERIDINE-1-CARBOXAMIDE; 4-(2-(4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-yl)-1-piperidinyl)-2-oxoethyl)-1-piperidinecarboxamide; SCHEMBL94653; BDBM14433; CHEBI:90678; DTXSID90870198;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5654 mL | 7.8269 mL | 15.6539 mL | |
| 5 mM | 0.3131 mL | 1.5654 mL | 3.1308 mL | |
| 10 mM | 0.1565 mL | 0.7827 mL | 1.5654 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02527707 | Completed Has Results | Drug: lonafarnib Drug: Ritonavir |
Chronic Delta Hepatitis | Eiger BioPharmaceuticals | September 2015 | Phase 2 |
| NCT02579044 | Enrolling by invitation | Drug: Everolimus and lonafarnib | Progeria | Boston Children's Hospital | December 2015 | Phase 1 Phase 2 |
| NCT05229991 | Active, not recruiting | Drug: Lonafarnib Drug: Ritonavir |
Hepatitis D, Chronic | Soroka University Medical Center | May 15, 2021 | Phase 3 |
| NCT00773474 | Terminated Has Results | Drug: Lonafarnib | Metastatic Breast Cancer | George Sledge | October 2008 | Phase 2 |