| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |

| 体外研究 (In Vitro) |
阿昔洛韦钠(3-100 µM;24-72 小时;Jurkat、U937 和 K562 白血病细胞)会降低细胞活力,其程度取决于剂量和时间 [1]。阿昔洛韦钠(10-100 µM;24-72 小时;Jurkat 细胞)提高亚 G1 亚二倍体峰值并抑制 DNA 合成,使细胞周期停止在 G2/M 和 S 期。 1]。阿昔洛韦钠(10-100 µM;24-72 小时;Jurkat 细胞)可激活 caspase-3 并使核 DNA 片段化,从而导致细胞凋亡 [1]。
|
|---|---|
| 体内研究 (In Vivo) |
阿昔洛韦钠(20 mg/kg;口服;每日 3 次;10 天)可防止皮肤损伤的形成,并将抗体的形成与 DTH 反应分开。 [3]
|
| 细胞实验 |
细胞活力测定[1]
细胞类型: Jurkat、U937 和 K562 白血病细胞 测试浓度: 3、10、30 和 100 µM 孵育持续时间:24、48和72小时 实验结果:证明细胞活力呈剂量和时间依赖性下降。 细胞凋亡分析[1] 细胞类型: Jurkat 细胞 测试浓度: 10 和 100 µM 孵育持续时间:24、48 和 72 小时 实验结果:增加 caspase-3 活性和核小体间 DNA 裂解。 细胞周期分析[1] 细胞类型: Jurkat 细胞 测试浓度: 10 和 100 µM 孵化持续时间:24、48和72小时 实验结果:24和48小时(hrs(小时)后,S期细胞的剂量依赖性积累))。 72小时后,亚G1亚二倍体峰出现剂量依赖性增加。 |
| 动物实验 |
Animal/Disease Models: Specific - Pathogen-free balb/c (Bagg ALBino) mouse (7 weeks old) infected with HSV-1 [3]
Doses: 20 mg/kg Route of Administration: po (po (oral gavage)) three times daily; for 10 days Experimental Results: Inhibition of skin The lesions develop and result in a dissociation between the DTH response and antibody production. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The oral bioavailability of acyclovir is 10-20% but decreases with increasing doses. Acyclovir ointment is <0.02-9.4% absorbed. Acyclovir buccal tablets and ophthalmic ointment are minimally absorbed. The bioavailability of acyclovir is not affected by food. Acyclovir has a mean Tmax of 1.1±0.4 hours, mean Cmax of 593.7-656.5ng/mL, and mean AUC of 2956.6-3102.5h/*ng/mL. The majority of acyclovir is excreted in the urine as unchanged drug. 90-92% of the drug can be excreted unchanged through glomerular filtration and tubular secretion. <2% of the drug is recovered in feces and <0.1% is expired as CO2. The volume of distribution of acyclovir is 0.6L/kg. The renal clearance of acyclovir is 248mL/min/1.73m2. The total clearance in neonates if 105-122mL/min/1.73m2. Absorption of acyclovir from the GI tract is variable and incomplete. 15-30% of an oral dose of the drug is absorbed. Some data suggest that GI absorption of acyclovir may be saturable; in a crossover study in which acyclovir was administered orally to healthy adults as 200 mg capsules, 400 mg tablets, or 800 mg tablets 6 times daily, the extent of absorption decreased with increasing dose, resulting in bioavailabilities of 20, 15, or 10%, respectively. ... This decrease in bioavailability appears to be a function of increasing dose, not differences in dosage forms. In addition, steady-state peak and trough plasma acyclovir concentrations were not dose proportional over the oral dosing range of 200-800 mg 6 times daily, averaging 0.83 and 0.46, 1.21 and 0.63, or 1.61 and 0.83 ug/ml for the 200, 400, or 800 mg dosing regimens, respectively. Peak plasma concentrations usually occur within 1.5-2.5 hours after oral administration. In a multiple dose study in neonates up to 3 months of age, IV infusion over 1 hour of 5, 10, or 15 mg/kg of acyclovir every 8 hours resulted in mean steady state peak serum concentrations of 6.8, 13.9, and 19.6 ug/ml, respectively, and mean steady state trough serum concentration of 1.2, 2.3, and 3.1 ug/ml, respectively. In another multiple dose study in pediatric patients, IV infusion over 1 hour of 250 or 500 mg/sq m of acyclovir every 8 hours resulted in mean steady state peak serum concentrations of 10.3 and 20.7 ug/ml, respectively. Acyclovir is widely distributed into body tissues and fluids including the brain, kidney, saliva, lung, liver, muscle, spleen, uterus, vaginal mucosa and secretions, cerebrospinal fluid, and herpetic vesicular fluid. The drug also is distributed into semen, achieving concentrations about 1.4 and 4 times those in plasma during chronic oral therapy at dosages of 400 mg and 1 g daily, respectively. The apparent volume of distribution of acyclovir is reported to be 32.4-61.8 liter/1.73 sq m in adults and 28.8, 31.6, 42, or 51.2-53.6 liter/1.73 sq m in neonates up to 3 months of age, children 1-2 years; 2-7 years; or 7-12 years of age, respectively. Acyclovir crosses the placenta. Limited data indicate that the drug is distributed into milk, generally in concentrations greater than concurrent maternal plasma concentrations, possibly via an active transport mechanism. For more Absorption, Distribution and Excretion (Complete) data for ACYCLOVIR (13 total), please visit the HSDB record page. Metabolism / Metabolites Acyclovir is <15% oxidized to 9-carboxymethoxymethylguanine by alcohol dehydrogenase and aldehyde dehydrogenase and 1% 8-hydroxylated to 8-hydroxy-acyclovir by aldehyde oxidase. Acyclovir is becomes acyclovir monophosphate due to the action of viral thymidine kinase. Acyclovir monophosphate is converted to the diphosphate form by guanylate kinase. Acyclovir diphosphate is converted to acyclovir triphosphate by nucleoside diphosphate kinase, pyruvate kinase, creatine kinase, phosphoglycerate kinase, succinyl-CoA synthetase, phosphoenolpyruvate carboxykinase and adenylosuccinate synthetase. Acyclovir is metabolized partially to 9-carboxymethoxymethylguanine and minimally to 8-hydroxy-9-(2-hydroxyethoxymethyl)guanine. In vitro, acyclovir also is metabolized to acyclovir monophosphate, diphosphate, and triphosphate in cells infected with herpes viruses, principally by intracellular phosphorylation of the drug by virus coded thymidine kinase and several cellular enzymes. Biological Half-Life The clearance of acyclovir varies from 2.5-3 hours depending on the creatinine clearance of the patient. The plasma half life of acyclovir during hemodialysis is approximately 5 hours. The mean half life in patients from 7 months to 7 years old is 2.6 hours. Plasma concentrations of acyclovir appear to decline in a biphasic manner. In adults with normal renal function, the half-life of acyclovir in the initial phase averages 0.34 hours and the half-life in the terminal phase averages 2.1-3.5 hours. In adults with renal impairment, both half-life in the initial phase and half-life in the terminal phase may be prolonged, depending on the degree of renal impairment. In a study in adults with anuria, the half-life in the initial phase of acyclovir averaged 0.71 hours. In several studies, the half-life in the terminal phase of acyclovir averaged 3,3.5, or 19.5 hours in adults with creatinine clearances of 50-80 or 15-50 ml/minute per 1.73 sq m or with anuria, respectively. In patients undergoing hemodialysis, the half-life in the terminal phase of acyclovir during hemodialysis averaged 5.4-5.7 hours. In neonates, the half-life of acyclovir depends principally on the maturity of renal mechanisms for excretion as determined by gestational age, chronologic age, and weight. In children older than 1 year of age, the half-life of the drug appears to be similar to that of adults. The half-life in the terminal phase averages 3.8-4.1, 1.9, 2.2-2.9, or 3.6 hours in neonates up to 3 months of age, children 1-2 years, 2-12 years, or 12-17 years of age, respectively. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Despite widespread use, there is little evidence that acyclovir when given orally causes significant liver injury. Serum enzyme levels generally do not change during oral acyclovir therapy. High dose intravenous administration of acyclovir is associated with renal dysfunction and thrombocytopenia, and occasionally with transient mild-to-moderate elevations in serum ALT levels, which have been asymptomatic and self-limited. There have rare instances of acute, clinically apparent liver injury reported that were attributed to acyclovir or valacyclovir (a prodrug of acyclovir with better oral absorption), but these have not been particularly convincing. Some degree of liver injury and even jaundice can occur during the course of herpes simplex or varicella zoster infection, and these complications could be mistaken for drug induced liver injury. Furthermore, in the reported cases, patients were receiving other medications and had other unlying comorbidities that may have been responsible for the liver injury. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Even with the highest maternal dosages, the dosage of acyclovir in milk is only about 1% of a typical infant dosage and would not be expected to cause any adverse effects in breastfed infants. Topical acyclovir applied to small areas of the mother's body away from the breast should pose no risk to the infant. Only water-miscible cream or gel products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking.[1] ◉ Effects in Breastfed Infants The mother of a 4-month-old infant noticed no adverse effects in her breastfed infant while she was taking an acyclovir dosage of 800 mg orally 5 times daily.[5] ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Acyclovir is 9-33% protein bound in plasma. Interactions Acyclovir has been used concomitantly with zidovudine ... without evidence of increased toxicity; however, neurotoxicity (profound drowsiness and lethargy), which recurred on rechallenge, has been reported in at least one patient with acquired immunodeficiency syndrome (AIDS) during concomitant therapy with the drugs. Neurotoxicity was evident within 30-60 days after initiation of IV acyclovir therapy, persisted with some improvement when acyclovir was administered orally, and resolved following discontinuance of acyclovir in this patient. This study reports the effects of a combination of azidothymidine plus acyclovir on both pluripotent (spleen colony forming units) and committed (granulocyte-macrophage colony forming units; erythroid burst forming units) murine hemopoietic progenitors. Administration of azidothymidine alone was associated with severe hematotoxicity, as shown by the marked decrease of all the hemopoietic progenitor populations tested, that is, spleen colony forming units, granulocyte-macrophage colony forming units, and erythroid burst forming units. This, however, was followed by a prompt recovery of hemopoiesis. Administration of acyclovir alone did not modify the hematological parameters studied, whereas the combined administration of azidothymidine and acyclovir led to changes in peripheral blood cells and bone marrow hemopoietic progenitors that were, on the whole, not significantly different from those observed with azidothymidine alone. Only the decrease in spleen colony forming units was significantly more severe, but their recovery was as rapid as that of the committed progenitors. Thus, in this experimental setting, the addition of acyclovir to azidothymidine does not appear to increase the hematotoxicity of the latter. The combined effect of acyclovir and chlorhexidine on the replication and DNA synthesis of herpes simplex virus was studied. Acyclovir and chlorhexidine showed synergism in the inhibition of the viral replication by enhancing in part the reduction of viral DNA synthesis. These data indicate that combined therapy with acyclovir and chlorhexidine might be beneficial for the control of intraoral herpetic infections. Acyclovir may decrease the renal clearance of other drugs eliminated by active renal secretion, such as methotrexate. For more Interactions (Complete) data for ACYCLOVIR (6 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse oral > 10,000 mg/kg LD50 Mouse ip 1000 mg/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antiviral Agents IV acyclovir sodium is used for the treatment of initial and recurrent mucocutaneous herpes simplex virus (HSV-1 and HSV-2) infections and the treatment of varicella-zoster infections in immunocompromised adults and children; for the treatment of severe first episodes of genital herpes infections in immunocompetent individuals; and for the treatment of HSV encephalitis and neonatal HSV infections. Acyclovir is used orally for the treatment of initial and recurrent episodes of genital herpes; for the acute treatment of herpes zoster (shingles, zoster) in immunocompetent individuals; and for the treatment of varicella (chickenpox) in immunocompetent individuals. Oral acyclovir is indicated in the treatment of initial episodes of genital herpes infection in immunocompetent and immunocompromised patients. Parenteral acyclovir is indicated in the treatment of severe initial episodes of genital herpes infection in immunocompetent patients and in patients who are unable to take (or absorb) oral acyclovir. /Included in US product labeling/ For more Therapeutic Uses (Complete) data for ACYCLOVIR (15 total), please visit the HSDB record page. Drug Warnings Parenteral acyclovir therapy can cause signs and symptoms of encephalopathy. ... Acyclovir should be used with caution in patients with underlying neurologic abnormalities and in patients with serious renal, hepatic, or electrolyte abnormalities or substantial hypoxia. The drug also should be used with caution in patients who have manifested prior neurologic reactions to cytotoxic drugs or those receiving intrathecal methotrexate or interferon. Acyclovir should be used with caution in patients receiving other nephrotoxic drugs concurrently since the risk of acyclovir-induced renal impairment and/or reversible CNS symptoms is increased in these patients. Adequate hydration should be maintained in patients receiving IV acyclovir; however, in patients with encephalitis, the recommended hydration should be balanced by the risk of cerebral edema. Because the risk of acyclovir-induced renal impairment is increased during rapid IV administration of the drug, acyclovir should be given only by slow IV infusion (over 1 hour). There are no adequate and controlled studies to date using acyclovir in pregnant women, and the drug should be used during pregnancy only when the potential benefits justify the possible risks to the fetus. Maternal Medication usually Compatible with Breast-Feeding: Acyclovir: Reported Sign or Symptom in Infant or Effect on Lactation: None. /from Table 6/ For more Drug Warnings (Complete) data for ACYCLOVIR (20 total), please visit the HSDB record page. Pharmacodynamics Acyclovir is a nucleoside analog that inhibits the action of viral DNA polymerase and DNA replication of different herpesvirus. Acyclovir has a wide therapeutic window as overdose is rare in otherwise healthy patients. |
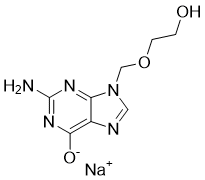
| 分子式 |
C8H10N5NAO3
|
|---|---|
| 分子量 |
247.18
|
| 精确质量 |
247.068
|
| CAS号 |
69657-51-8
|
| 相关CAS号 |
Acyclovir;59277-89-3;Acyclovir alaninate;84499-64-9
|
| PubChem CID |
135398513
|
| 外观&性状 |
Crystals from methanol
Crystals from ethanol White, crystalline powder |
| 沸点 |
613.1ºC at 760 mmHg
|
| LogP |
0.099
|
| tPSA |
122.14
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
16
|
| 分子复杂度/Complexity |
308
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C1=NC2=C([N]1COCCO)N=C(N=C2[O-])N.[Na+]
|
| InChi Key |
MKUXAQIIEYXACX-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C8H11N5O3/c9-8-11-6-5(7(15)12-8)10-3-13(6)4-16-2-1-14/h3,14H,1-2,4H2,(H3,9,11,12,15)
|
| 化学名 |
2-amino-9-(2-hydroxyethoxymethyl)-1H-purin-6-one
|
| 别名 |
Aciclovir sodiumAcyclovir sodium
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0456 mL | 20.2282 mL | 40.4563 mL | |
| 5 mM | 0.8091 mL | 4.0456 mL | 8.0913 mL | |
| 10 mM | 0.4046 mL | 2.0228 mL | 4.0456 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
The Bioequivalence Study of Acyclovir 800 mg Tablet in Healthy Thai Volunteers Under Fasting Conditions
CTID: NCT06228430
Phase: Phase 1 Status: Not yet recruiting
Date: 2024-01-29