| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
AMPK
|
|---|---|
| 体外研究 (In Vitro) |
AICA-核糖苷/AICA riboside(5-氨基咪唑-4-甲酰胺-1-β-D-呋喃核糖苷)已被广泛用于细胞中,以激活AMPK(AMP活化蛋白激酶),AMPK是一种参与细胞能量平衡的代谢传感器。在本研究中,我们研究了AICA核糖苷对线粒体氧化的影响;磷酸化。发现AICA核糖苷能剂量依赖性地抑制分离的大鼠肝细胞的寡霉素敏感JO2(耗氧率)。当AICA核糖苷浓度>0.1 mM时,也观察到P(i)(无机磷酸盐)、ATP、AMP和总腺嘌呤核苷酸含量降低。有趣的是,在缺乏α1和α2 AMPK催化亚基的小鼠肝细胞中,与野生型小鼠相比,基础JO2和几种线粒体蛋白的表达显著降低,表明线粒体生物合成受到干扰。然而,AICA核糖苷对JO2的抑制作用在突变小鼠中仍然存在,因此显然不是由AMPK介导的。在透性肝细胞中,这种抑制作用不再明显,表明这可能是由于细胞内Z核苷酸的积累和/或腺嘌呤核苷酸和P(i)的损失。ZMP确实通过对呼吸链复合物I的直接作用抑制了分离的大鼠线粒体的呼吸。此外,在与果糖一起孵育的细胞中,AICA核糖苷对JO2的抑制作用也得到了增强,以耗尽腺嘌呤核苷酸和P(I)。我们得出结论,AICA核糖苷通过AMPK非依赖性机制抑制细胞呼吸,这可能是由于细胞内P(i)耗竭和ZMP积累共同作用的结果。我们的数据还表明,AICA核糖苷的细胞效应不一定是由AMPK激活引起的,对它们的解释应该谨慎。[1]
5-氨基咪唑-4-甲酰胺核糖苷(AICA riboside/AICA-核糖苷;阿卡德新)激活完整细胞中的AMP活化蛋白激酶(AMPK),据报道对哺乳动物中枢神经系统具有保护作用。在大鼠大脑皮质脑切片中,AMPK被代谢应激(缺血>缺氧>多糖血症)和AICA核糖苷(0.1-10mm)激活。平衡核苷转运抑制剂大大减弱了AICA核苷对AMPK的激活。AICA-核糖苷还抑制了大鼠海马CA1区的兴奋性突触传递,这种传递被腺苷A1受体拮抗剂阻止,并被腺苷脱氨酶逆转。然而,AICA-核糖苷既不是腺苷脱氨酶的底物,也不是腺苷受体的激动剂。我们得出结论,代谢应激和AICA核糖核苷都会刺激哺乳动物大脑中的AMPK活性,但AICA核糖苷还有一个额外的作用,即与腺苷竞争核苷转运蛋白的摄取。这导致细胞外腺苷增加,随后腺苷受体激活。AICA核糖苷的神经保护作用可能是通过这种机制介导的,也可能是通过AMPK激活介导的。因此,在将AICA核糖苷的作用归因于AMPK激活时应谨慎,特别是在抑制腺苷再摄取具有生理后果的系统中[2]。 |
| 酶活实验 |
与腺苷脱氨酶作用相关的pH变化[2]
腺苷(10、30和100 µm)和AICA核糖苷(1、3和10 mm)制备为20 生理盐水(0.9%氯化钠;pH 5.72). 将腺苷脱氨酶(0.02单位)一式三份加入其中,用Jenway 3310 pH计测量所得pH变化。在研究AICA-核糖苷对腺苷脱氨酶代谢腺苷能力的影响的实验中,30 µm腺苷,来自5μm的储备溶液 向1、3和10的溶液中加入三份mm mm AICA核糖苷,含有0.02单位的腺苷脱氨酶。 市售AICA核糖苷的纯度/AICA riboside[2] 我们检查了本研究中使用的AICA核糖苷是否有可能被腺苷或可分解为腺苷的腺嘌呤核苷酸污染的迹象。使用了两种独立的HPLC技术,即上述用于组织核苷酸测定的技术,以及第二种技术,其中AICA核糖苷溶液被乙烯化,并用ThermoFenigan FL3000扫描荧光检测器检测成分。用任何一种技术(数据未显示)测量的AICA-核糖苷溶液中都没有可检测的污染物,排除了所述结果可能归因于腺苷或腺嘌呤核苷酸存在的可能性。 |
| 细胞实验 |
完整和透性肝细胞以及分离线粒体线粒体耗氧率的测定[1]
将大鼠或小鼠肝细胞(7-8mg干细胞·ml-1)在37°C的振荡水浴中,在含有2ml Krebs–Ringer碳酸氢盐钙缓冲液[120mM NaCl、4.8mM KCl、1.2mM KH2PO4、1.2mM MgSO4、24mM NaHCO3和1.3mM CaCl2(pH 7.4)]的封闭小瓶中孵育,并补充指定浓度的底物和AICA核糖苷,并与含有O2/CO2的气相平衡(19:1)。在指定时间,将细胞悬浮液再次用O2/CO2饱和1分钟,并立即转移到配备有Clark氧电极的搅拌式氧气计容器中。在连续添加0.5μM寡霉素、150μM DNP(2,4-二硝基苯酚)、0.15μg/ml抗霉素和1 mM TMPD(N,N,N′,N′-四甲基-1,4-苯二胺)加5 mM抗坏血酸之前和之后,在37°C下测量JO2(耗氧率)。 对于线粒体实验,将分离的大鼠线粒体(1 mg蛋白质·ml−1)在37°C下在含有2 ml KCl培养基(如上所述)的血氧计容器中在1 mMAICA核糖苷或指定浓度的ZMP和ZTP存在下孵育。平衡30秒后,在谷氨酸/苹果酸或琥珀酸/苹果酸/鱼藤酮存在的情况下监测线粒体呼吸速率,如上所述用于透性肝细胞。 核苷酸和Pi浓度以及糖异生和酮生的测定[1] 孵育30分钟后,将细胞悬浮液样品在冰冷的25 mM HClO4[5%(w/v)]/25 mM EDTA中淬灭,并离心(13000 g,2分钟)。中和上清液,用高效液相色谱法测定腺嘌呤核苷酸、ZMP和AICA核糖苷的浓度。为了测量细胞内Pi,将0.7ml细胞悬浮液样品通过硅油层离心(13000g,1分钟)至0.25ml HClO4[10%(w/v)]/25mM EDTA中。用比色法测量Pi。对于糖异生和酮生成测量,将细胞悬浮液样品在冰冷的HClO4[5%(w/v)]中淬灭,离心(13000 g,2分钟),中和上清液。如前所述,使用酶法结合NADH的分光光度测定法测量葡萄糖、乙酰乙酸盐和β-羟基丁酸盐的浓度。 大脑皮质切片的治疗[2] 将大脑皮层切片转移到放置在水浴中的孵化室中,将其加热至33-34°C。大约2.5-3之后 h、 将一些切片转移到相同的实验室中,这些实验室含有AICA核糖苷(0.1-10 mm)或AICA核糖糖苷以及摄取抑制剂双嘧达莫和硝基苄硫肌苷的组合(DIPY/NBTI;5 µm和1 μm)。此外,为了诱导代谢应激,切片被转移到没有葡萄糖的腔室中(低血糖;葡萄糖被10 mm蔗糖)或氧气(缺氧;用95%N2/5%CO2鼓泡)或缺乏葡萄糖和氧气(缺血)。将用作时间对照的切片转移到含有标准aCSF的相同腔室中。在适当的时候,切片在液氮中快速冷冻,并储存在-80°C下。 |
| 动物实验 |
Slice preparation [2]
Sprague–Dawley rats of either sex, aged 16–23 days, were killed by cervical dislocation. The brain was rapidly removed and placed in ice-cold artificial cerebro-spinal fluid (aCSF) containing 11 mm Mg2+ wherein 400-µm cerebrocortical slices, comprising sagittal sections of hippocampus and overlying neocortex, were cut with a Vibratome, as previously described (Dale et al. 2000). Slices were placed in an incubation chamber comprising a nylon mesh within a beaker of continuously circulating, oxygenated (95% O2/5% CO2) standard aCSF (1 mm Mg2+) and kept at room temperature for at least 1 h before use. The composition of the aCSF solution was: NaCl 124 mm; KCl 3 mm; CaCl2 2 mm; NaHCO3 26 mm; NaH2PO4 1.25 mm; d-glucose 10 mm; MgSO4 1 mm; pH 7.4 with 95% O2/5% CO2. Treatment of cerebrocortical slices [2] Cerebrocortical slices were transferred to incubation chambers placed in a water bath where they were warmed to 33–34°C. After approximately 2.5–3 h, some slices were transferred to identical experimental chambers that contained either AICA riboside (0.1–10 mm) or AICA riboside plus a combination of uptake inhibitors dipyridamole and nitrobenzylthioinosine (DIPY/NBTI; 5 µm and 1 µm, respectively). Additionally, to induce metabolic stress, slices were transferred to chambers that were devoid of glucose (aglycaemia; glucose substituted with 10 mm sucrose) or oxygen (hypoxia; bubbled with 95% N2/5% CO2) or devoid of both glucose and oxygen (ischaemia). Slices used as time controls were transferred to identical chambers containing standard aCSF. At appropriate times, slices were snap-frozen in liquid nitrogen and stored at − 80°C. Generation of AMPKα1α2LS−/− knockout mice [1] To obtain a deletion of both catalytic subunits in the liver (AMPKα1α2LS−/−), we first generated a liver-specific AMPKα2-null mouse (AMPKα2−/−) by crossing floxed AMPKα2 mice and an AlfpCre transgenic line expressing the Cre recombinase under the control of the albumin and α-fetoprotein regulatory elements. We then produced a liver-specific AMPKα1α2LS−/− mouse on an AMPKα1−/− background by crossing liver-specific AMPKα2−/− mice with AMPKα1−/− mice. Mice were genotyped using PCR on DNA extracted from a tail biopsy using specific primers for the Cre transgene and for the floxed AMPKα2, the deleted AMPKα1 and the wild-type AMPKα1 gene. |
| 参考文献 |
[1]. AMP-activated protein kinase-independent inhibition of hepatic mitochondrial oxidative phosphorylation by AICA riboside. Biochem J. 2007 Jun 15;404(3):499-507.
[2]. AICA riboside both activates AMP-activated protein kinase and competes with adenosine for the nucleoside transporter in the CA1 region of the rat hippocampus. J Neurochem. 2004 Mar;88(5):1272-82. |
| 其他信息 |
AICA ribonucleotide is a 1-(phosphoribosyl)imidazolecarboxamide that is acadesine in which the hydroxy group at the 5' position has been converted to its monophosphate derivative. It has a role as a cardiovascular drug, a plant metabolite, a human metabolite, an Escherichia coli metabolite, a Saccharomyces cerevisiae metabolite and a mouse metabolite. It is a 1-(phosphoribosyl)imidazolecarboxamide and an aminoimidazole. It is functionally related to an acadesine. It is a conjugate acid of a 5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamide(2-).
5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) is an intermediate in the generation of inosine monophosphate and analog of adenosine monophosphate (AMP) that is capable of stimulating AMP-dependent protein kinase (AMPK) activity. AICAR has been used clinically to treat and protect against cardiac ischemic injury. The drug was first used in the 1980s as a method to preserve blood flow to the heart during surgery and is currently of interest as a potential treatment for diabetes by increasing the metabolic activity of tissues by changing the physical composition of muscle. AICA ribonucleotide has been reported in Arabidopsis thaliana, Homo sapiens, and other organisms with data available. 5-Amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamide is a metabolite found in or produced by Saccharomyces cerevisiae. The present investigation was undertaken to examine the activation of AMPK in mammalian brain by both metabolic stress and the exogenous activator AICA riboside (Acadesine). Furthermore, given that AICA riboside has been reported to protect both peripheral organs and the brain we sought to determine the effects of AICA riboside in a physiological context, that of excitatory synaptic transmission in the rodent hippocampus. Two aspects of AICA riboside, i.e. its (unspecified) action as an adenosine regulating agent (Mullane 1993) and as an activator (Corton et al. 1995) of AMPK (a key intracellular enzyme involved in the cellular response to metabolic stress), raises the possibility that the protective action of AICA riboside might reflect its effects on extracellular adenosine and activation of AMPK. One or other, or a combination, of these actions could explain the protection of peripheral organs and tissue in a wide variety of experimental models of ischaemia/reperfusion injury (Nawarskas 1999; Matot and Jurim 2001), trauma (Davis et al. 2000), shock (Ragsdale and Proctor 2000) and sepsis (Melton et al. 1999), and how, in humans, AICA riboside improved outcome after coronary artery bypass surgery, reducing the incidence of early cardiac death and myocardial infarction (Mangano 1997). However, it is clear that AMPK mediates the AICA riboside-induced improvement of glucose homeostasis in animal models of diabetes (Halseth et al. 2002; Song et al. 2002), as the antidiabetes drug, metformin, also activates AMPK in vivo (Zhou et al. 2001; Fryer et al. 2002; Hawley et al. 2002) and in skeletal muscle of diabetic humans (Musi et al. 2002). Similarly, in the brain, AICA riboside exerts protective effects in attenuating neuropathology and locomotor deficits associated with 5-min bilateral carotid artery occlusion in the gerbil (Clough-Helfman and Phillis 1990) and specifically inhibiting homocysteine thiolactone-induced seizures in mice (Marangos et al. 1990), although not pentylenetetrazol, caffeine, picrotoxin or bicuculline methiodide-induced seizures (Marangos et al. 1990; Zhang et al. 1993). Its protective influences have been attributed to activation of adenosine receptors, inhibition of adenosine uptake or metabolism, or to scavenging of free radicals, all of which are known to be neuroprotective (Lipton 1999; de Mendonça et al. 2000). Indeed, a low concentration of AICA riboside (20 µm) has also been shown to potentiate adenosine release from rat cortical slices induced by activation of kainate receptors, but not by α-amino-3-hydroxy-5-methyl-4-isoxasole propionic acid (AMPA) or N-methyl-d-aspartate (NMDA) receptors (White 1996). However, a role for AMPK in the protection of brain tissues cannot be excluded since activation of AMPK via chronic exposure (48 h) to 200 µm AICA riboside reduced ceramide-induced apoptosis in cultured cortical astrocytes (Blazquez et al. 2001). Moreover, 1 h pre-treatment of hippocampal neuronal cultures with very low (10–100 µm), but not higher (0.5–1 mm) concentrations of AICA riboside has been shown to offer complete protection against toxicity induced by subsequent 24 h exposure to glucose deprivation, cyanide, glutamate and amyloid β peptide (Culmsee et al. 2001). This protection was concomitant with an activation of AMPK as measured by increased phosphorylation of the α subunit using a phospho-specific antibody. Interestingly, protection against glucose withdrawal was observed in the presence of DPCPX, a competitive adenosine A1 receptor antagonist, and could be prevented with antisense oligonucleotides directed against the catalytic α1 and α2 subunits, which suggested a direct involvement of AMPK (Culmsee et al. 2001). Clearly, the combined actions of AICA riboside via adenosine A1 receptors in intact neuronal tissue, which can limit seizure activity (Dunwiddie 1999) and ischaemic brain damage (de Mendonça et al. 2000), and the activation of AMPK, which may additionally provide astrocytic ketone bodies for neuronal oxidative metabolism (Blazquez et al. 1999), endows AICA riboside with several attractive qualities as a potential agent for use in acute or chronic dysfunctions of the mammalian CNS. In the present study we have shown that AMPK can be activated by metabolic stress induced by hypoxia, aglycaemia and ischaemia in intact brain tissue, with the extent of activation reflecting the severity of the insult. The apparent decline in the activity of AMPK after prolonged ischaemia, may reflect widespread neuronal pathology and/or severe depletion of intracellular ATP, which showed little signs of recovery even after 30 min in oxygen/glucose-rich aCSF. Reductions in ATP levels would render the upstream kinase (AMPKK) less able to phosphorylate AMPK and contribute to a decline in AMPK activity. Indeed, transient increases in AMPK activity followed by marked decreases have also been observed during ischaemia in intact rat hearts perfused in vitro (Marsin et al. 2000) or hypoxia or treatment with the ATP synthesis inhibitor, oligomycin, in monocytes (Marsin et al. 2002). As in non-neuronal tissue, AICA riboside activated AMPK via intracellular production of ZMP whilst not affecting ATP/ADP ratios. That conversion of AICA riboside to ZMP requires transport of AICA riboside via the equilibrative adenosine transporter is a novel finding of this study clearly indicated by the inhibitory effects on ZMP accumulation and AMPK activity using the nucleoside transport inhibitors DIPY and NBTI. Moreover, the functional implications of AICA riboside transport for neuronal activity is to result in an increase in extracellular adenosine, a process reversed by exogenous adenosine deaminase, which may in itself be inhibited by AICA riboside, thereby further increasing levels of extracellular adenosine. The accumulation of extracellular adenosine is capable of causing strong inhibition of excitatory synaptic transmission, likely via inhibition of glutamate release via pre-synaptic adenosine A1 receptors. We addressed whether AICA riboside was an agonist at adenosine receptors using two different reporter gene assays which showed that AICA riboside is devoid of efficacy at A1 receptors (as well as A2A and A2B receptors) in yeast and against A3 receptors expressed in CHO cells. Given the use of all four adenosine receptors and two assay systems, it makes it unlikely, although not impossible, that the lack of effect of AICA riboside in both could be attributable to inactivation of all four adenosine receptors by some phosphorylation event initiated by AMPK activation. Instead, the simplest interpretation of the reporter gene assay data is that AICA riboside is not an agonist at adenosine receptors. The consequences of AICA riboside inhibition of adenosine re-uptake would not be specific to A1 receptors, but instead would result in activation of the predominant adenosine receptors in that particular region of the brain (for example A2A receptors in the striatum). This could lead to the situation where interactions between A1, A2A and A3 receptors might result in the desensitisation of A1 receptor-mediated inhibition (Dunwiddie et al. 1997; Lopes et al. 2002). These interactions may have deleterious consequences (Ribeiro et al. 2002) and might explain the mixed results obtained in different models of seizure activity. Nonetheless, previous studies have shown that inhibition of adenosine uptake can raise extracellular adenosine and reduce neuropathology in a variety of experimental models of CNS disorders (de Mendonça et al. 2000). For example, propentofylline, given intraperitoneally after combined hypoxia/ischaemia in newborn rat pups, reduced the volume of infarcted tissue and resulted in better histological measures in striatum, thalamus, hippocampus and cortex (Gidday et al. 1995) and potentiated the neuroprotective ischaemic preconditioning phenomenon in gerbil hippocampus (Kawahara et al. 1998). Indeed, in a recent clinical trial a combination of aspirin and DIPY (a clinically used antiplatelet drug), reduced the recurrence of stroke, whilst DIPY has been shown to elevate plasma levels of adenosine in humans, a factor which may have contributed to the favourable outcome (Picano and Abbracchio 1998). Thus it would seem that an additional facet of the neuroprotective potential of AICA riboside may reside in its ability to elevate extracellular adenosine. In conclusion, our findings that AICA riboside (while activating AMPK) also exerts effects by elevating extracellular levels of adenosine in intact neuronal tissue, should encourage caution in the interpretation of results obtained with this agent. It remains to be determined which of these two mechanisms of AICA riboside action is more important in providing neuroprotection in vivo. Nonetheless, the existence of these two mutually beneficial properties potentially provides a valuable synergism that could be exploited therapeutically in disorders of the mammalian CNS.[2] |
| 分子式 |
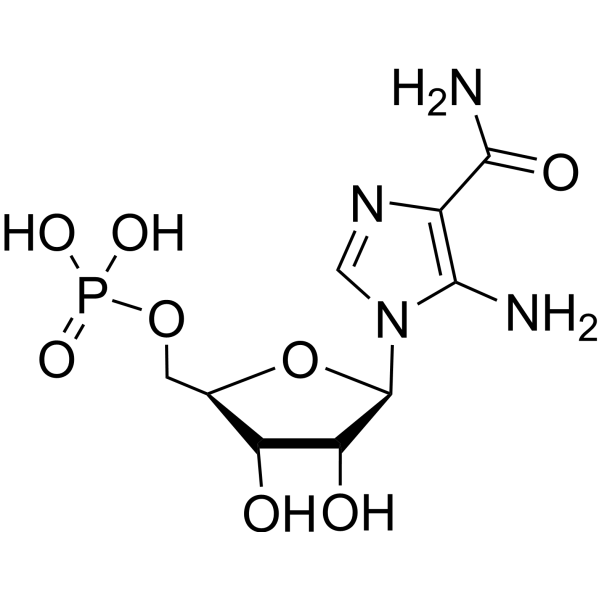
C9H15N4O8P
|
|---|---|
| 分子量 |
338.2112
|
| 精确质量 |
338.063
|
| 元素分析 |
C, 31.96; H, 4.47; N, 16.57; O, 37.84; P, 9.16
|
| CAS号 |
3031-94-5
|
| 相关CAS号 |
3031-94-5; 681006-28-0 (phosphate)
|
| PubChem CID |
65110
|
| 外观&性状 |
White to light brown solid powder
|
| 密度 |
2.3g/cm3
|
| 沸点 |
845.3ºC at 760 mmHg
|
| 熔点 |
198-202ºC dec.
|
| 闪点 |
465ºC
|
| 折射率 |
1.831
|
| LogP |
-3.8
|
| tPSA |
213.19
|
| 氢键供体(HBD)数目 |
6
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
475
|
| 定义原子立体中心数目 |
4
|
| SMILES |
C1=NC(=C(N1[C@H]2[C@@H]([C@@H]([C@H](O2)COP(=O)(O)O)O)O)N)C(=O)N
|
| InChi Key |
NOTGFIUVDGNKRI-UUOKFMHZSA-N
|
| InChi Code |
InChI=1S/C9H15N4O8P/c10-7-4(8(11)16)12-2-13(7)9-6(15)5(14)3(21-9)1-20-22(17,18)19/h2-3,5-6,9,14-15H,1,10H2,(H2,11,16)(H2,17,18,19)/t3-,5-,6-,9-/m1/s1
|
| 化学名 |
[(2R,3S,4R,5R)-5-(5-amino-4-carbamoylimidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate
|
| 别名 |
AICA ribonucleotide; 3031-94-5; Z-nucleotide; aminoimidazole carboxamide ribonucleotide; 5'-Phosphoribosyl-5-amino-4-imidazolecarboxamide; 5-amino-4-imidazolecarboxamide ribotide; Acadesine 5'-monophosphate; AICA-ribonucleotide;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9567 mL | 14.7837 mL | 29.5674 mL | |
| 5 mM | 0.5913 mL | 2.9567 mL | 5.9135 mL | |
| 10 mM | 0.2957 mL | 1.4784 mL | 2.9567 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。