| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Unlike baclofen, absorption of R-baclofen(arbaclofen) is not limited to the upper small intestine. The ability of arbaclofen to be absorbed throughout the gastrointestinal tract allowed for the development of the sustained release formulation, arbaclofen placarbil (AP). In one study of AP absorption in 10 healthy volunteers, one 20mg oral dose of AP, in the presence of food, resulted in a Tmax of 5.05h. The oral bioavailability of R-baclofen in rats when AP was dosed at 10mg/kg was 44 ± 12%, and when dosed at 1mg/kg, oral bioavailability was 68 ± 6%. In monkeys and dogs, the oral bioavailability of R-baclofen when AP was orally dosed was high: 94 ± 16%, and 92 ± 7%, respectively. In comparison, when oral R-balofen was dosed oral bioavailability was 39 ± 21% in monkeys and 49 ± 20% in dogs. Colonic absorption studies measuring R-baclofen bioavailability post intracolonic dosing in rats and monkeys, have revealed low bioavailability with the administration of R-baclofen (7 ± 3% and 3 ± 2%, respectively), and significantly higher R-baclofen bioavailability with intracolonic dosing of AP suspension ( 37 ± 9% and 37 ± 15%, in rats and monkeys respectively). Intracolonic dosing of AP suspension also resulted in high biolavailability of R-baclofen in dogs (77 ± 23%). Absorption throughout the intestine is both passive and active and occurs via the monocarboxylate type 1 transporter. 84-88% renal elimination as R-baclofen. Less than 1% fecal elimination. (2) Radioactive labeling has shown AP to be widely distributed throughout the body. Tissue distribution occurs mostly to the kidneys and liver. Blood clearance of an IV bolus of AR in rats resulted in a total blood clearance of 15.81 ± 10.2 L/h/kg in rats. In comparison, blood clearance of an IV bolus of R-baclofen in rats, monkeys, and dogs, resulted in half lives ranging from 1.6-3.4hours, with total blood clearances reported to be 0.51± 0.13L/h/kg in rats, 0.31±0.11L/h/kg in monkeys, and 0.24L±0.01L/h/kg in dogs. (2) In studied utilizing radioactive tracers attached to R-baclofen, 97% of radioactivity was recovered in the urine. Metabolism / Metabolites In experimental studies using human liver S9 Arbaclofen placarbil was not shown to be a substrate for CYP1A2, CYP2C19, CYP2D6, CYP2E1, and CYP3A4. Arbaclofen placarbil, the acyloxyalkyl carbamate prodrug of R-arbaclofen, is believed to undergo hydrolysis by the esterase enzyme human carboxylesterase-2 into the parent amine, R-baclfen. Carbon dioxide, isobutyric acid, isobutyraldehyde, are also expected to be produced in equimolar quantities. The productions of isobutyric acid has been confirmed in vitro untilizing mass spectrometry and gas chromatography. Biological Half-Life IV bolus administration of AP to rats showed that AP was converted to R-baclofen with a half life of 6 minutes. |
|---|---|
| 参考文献 |
: Erickson CA, Veenstra-Vanderweele JM, Melmed RD, McCracken JT, Ginsberg LD, Sikich L, Scahill L, Cherubini M, Zarevics P, Walton-Bowen K, Carpenter RL, Bear MF, Wang PP, King BH. STX209 (arbaclofen) for autism spectrum disorders: an 8-week open-label study. J Autism Dev Disord. 2014 Apr;44(4):958-64. doi: 10.1007/s10803-013-1963-z. PubMed PMID: 24272415.
|
| 其他信息 |
Arbaclofen Placerbil is a prodrug of Arbaclofen, which is a selective gamma-amino-butyric acid type B receptor agonist and the R-enantiomer of baclofen. It was discovered, and has been patented by XenoPort as a new chemical entity with an improved pharmacokinetic profile compared to baclofen, which allows for sustained release properties. Arbaclofen Placerbil was believed to have therapeutic potential in treating gastroesophogeal reflux disease (GERD) and plasticity; however due to discouraging clinical trial results, the drug was abandoned by XenoPort in 2011 for the treatment of GERD. On May 20th, 2013, XenoPort announced plans to terminate the development of Arbaclofen Placerbil for the treatment of multiple sclerosis.
Drug Indication Investigated for the treatment of spasticity in multiple sclerosis, acute back spasms, and GERD. Mechanism of Action R-baclofen is postulated to aid in spasticity by acting as an agonist of the inhibitory gamma aminobutyric acid neurotransmission pathway. |
| 分子式 |
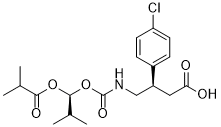
C19H26CLNO6
|
|---|---|
| 分子量 |
399.8658452034
|
| 精确质量 |
399.144
|
| CAS号 |
847353-30-4
|
| 相关CAS号 |
847353-30-4 (Arbaclofen placarbil); 69308-37-8 (Arbaclofen); 63701-55-3 (Arbaclofen hydrochloride)
|
| PubChem CID |
11281011
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
545.1±50.0 °C at 760 mmHg
|
| 闪点 |
283.5±30.1 °C
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
| 折射率 |
1.523
|
| LogP |
4.39
|
| tPSA |
105.42
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
502
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CC(C)C(OC(=O)C(C)C)OC(=O)NCC(CC(=O)O)C1=CC=C(C=C1)Cl
|
| InChi Key |
JXTAALBWJQJLGN-KSSFIOAISA-N
|
| InChi Code |
InChI=1S/C19H26ClNO6/c1-11(2)17(24)26-18(12(3)4)27-19(25)21-10-14(9-16(22)23)13-5-7-15(20)8-6-13/h5-8,11-12,14,18H,9-10H2,1-4H3,(H,21,25)(H,22,23)/t14-,18-/m0/s1
|
| 化学名 |
(R)-3-(4-chlorophenyl)-4-((((S)-1-(isobutyryloxy)-2-methylpropoxy)carbonyl)amino)butanoic acid
|
| 别名 |
XP19986 XP-19986 XP 19986 Arbaclofen placarbil.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5008 mL | 12.5041 mL | 25.0081 mL | |
| 5 mM | 0.5002 mL | 2.5008 mL | 5.0016 mL | |
| 10 mM | 0.2501 mL | 1.2504 mL | 2.5008 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。