| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Complement 5a receptor (IC50: 0.1 nM)
|
|---|---|
| 体外研究 (In Vitro) |
CCX168阻断了U937细胞以及新分离的人类中性粒细胞中的C5a结合、C5a介导的迁移、钙动员和CD11b上调。CCX168在人体血液中存在时保持高效力。
CCX168取代了与人髓系细胞系(U937)上的C5aR结合的[125I]-C5a,其效力(IC50值)为0.1 nM(图3A)。使用了两种CCX168效力的测量方法,IC50(对一半最大激动剂浓度的50%抑制)和剂量比或A2(在C5a活性中产生2倍右移的CCX168浓度)(图3B和3C)。CCX168以0.2nM的效力(A2)抑制C5a介导的U937细胞的趋化性。在钙动员测定中向U937细胞中添加CCX168抑制C5a,其效力(A2)为0.1nM(图3D)。CCX168抑制100%人血浆中U937细胞的趋化性(图3E),在存在α1-酸性糖蛋白的情况下没有失去作用(图3F)。此外,CCX168在所用的任何测定中都没有显示出任何激动剂活性(细胞质钙通量、趋化性或CD11b上调)。CCX168对C5aR是选择性的,用C5aR相关受体C5L2、C3aR、ChemR23、GPR1和FPR1、一组18个趋化因子受体、一组54个药理学相关受体和细胞色素P450酶1A2、2C9、2C19、2D6、3A4测得没有活性(IC50>5000nM)(详细信息见S1和S2表)。此外,如在膜片钳测定中测得的,CCX168不抑制hERG钾离子通道(IC50<5000nM)。[1] CCX168竞争性和选择性地阻断纯化的人类中性粒细胞中C5a诱导的钙动员,IC50值为0.2 nM(图4A)。使用纯化的人血单核细胞也获得了类似的结果。CCX168还以0.2 nM的IC50抑制[125I]-C5a与人中性粒细胞上C5aR的结合(图4B)。此外,CCX168以1.7nM的A2抑制新采集的人类血液中中性粒细胞的C5a介导的趋化性(图4C)。CCX168以3.0nM的A2抑制C5a诱导的人全血中性粒细胞上整合素CD11b水平的增加(图4D)。此外,CCX168抑制C5a诱导的活性氧从分离的中性粒细胞中释放,并能够完全阻断这些中性粒细胞的呼吸爆发(图4E)。取自类风湿性关节炎(RA)或骨关节炎(OA)诱导的人类血液白细胞趋化性受试者的滑液样本[24];CCX168显著降低了这种反应,表明两种类型的滑膜样品都含有活性C5a(图4F)。 |
| 体内研究 (In Vivo) |
CCX168在体外和离体趋化性测定中有效阻断迁移,并阻断C5a介导的中性粒细胞血管内皮边缘化。CCX168在食蟹猴的迁移和中性粒细胞边缘测定中有效。这种彻底的体外和临床前表征使CCX168能够进入临床,并在48名健康志愿者的1期临床试验中测试其安全性、耐受性、药代动力学和药效学特征。CCX168在宽剂量范围(1至100mg)内表现出良好的耐受性,并且表现出剂量依赖性的药代动力学。每天两次的30 mg CCX168口服剂量在一整天中阻断了C5a诱导的循环中性粒细胞中CD11b的上调94%或更高,显示出基本上完全的靶向覆盖。该剂量方案正在抗中性粒细胞细胞质抗体相关血管炎患者的临床试验中进行测试[1]。
口服CCX168,一种人C5aR/CD88的小分子拮抗剂,改善了表达人C5aR/CD88的小鼠中抗MPO诱导的NCGN。这些观察结果表明,阻断C5aR/CD88可能对ANCA相关血管炎和GN患者具有治疗益处[2]。 |
| 酶活实验 |
体外测定如前所述进行趋化性、钙动员和放射性配体结合测定。如所述进行呼吸爆发测定。CCX168影响C5a介导的迁移的能力是通过量化浓度曲线中向右移动的程度来确定的。这表示为“A”值。例如,A2值表示CCX168的浓度,其导致C5a介导的作用的剂量-反应曲线向右移动两倍,并且与竞争性拮抗剂如CCX168 50%的受体占有率相关。如所述,使用以下方程进行功能测定的效价计算(A2)[1]。
|
| 细胞实验 |
人U937细胞在RPMI-1640培养基中培养,该培养基补充有10%胎牛血清并在使用前18小时向细胞中加入二丁基cAMP(0.5mM)。THP-1、HEK293、MOLT4、Baf3和MDA-MB435细胞从ATCC获得并根据它们的建议生长。L1.2细胞由Eugene Butcher博士授权。如所述培养活化的人T淋巴细胞。将所有血液收集到EDTA中作为抗凝血剂。从健康志愿者身上采集人类全血,并在两小时内使用。使用标准密度梯度分离方法从人全血中分离中性粒细胞。食蟹猴全血来自加州国家灵长类动物研究中心,采集后4小时内使用[1]。
|
| 动物实验 |
For in vivo assays, CCX168 was formulated in PEG-400/solutol-HS-15 (70:30)
C5a-induced leukopenia in human C5aR knock-in mice Human C5aR knock-in mice were dosed with vehicle (PEG-400/solutol-HS15 70:30, 5 mL/kg) or CCX168 by oral gavage. One hour after dosing, C5a (20 μg/kg, 0.1 mL dose volume) was injected intravenously and blood samples collected from retro-orbital eye bleeds. Blood leukocyte levels were analyzed by flow cytometry. C5a-induced neutropenia in cynomolgus monkeys All experiments performed in cynomolgus monkeys were performed at Covance Research Products with the approval of Covance Research Products Animal Care and Use Committee and in compliance with the Guide for the Care and Use of Laboratory Animals essentially as previously described[1]. Humanization of Mice with Human C5aR[2] Standard homologous recombination techniques were used to create mice with the murine C5a receptor replaced with the human C5a receptor. These mice had a mixed genetic background of 129S6 and C57BL/6. In addition to standard confirmation by genotyping, the effectiveness of replacement of the mC5aR with the hC5aR was tested by determining leukocyte expression of mC5aR versus hC5aR on peripheral blood leukocytes by flow cytometry, and by measuring CCX168 suppression of human C5a-induced chemotaxis of thioglycollate-induced peritoneal leukocytes from mC5aR versus hC5aR mice. CCX168 was formulated in polyethylene glycol 400/Solutol (70/30). Response to human C5a of hC5aR knock-in mouse leukocytes was tested in vitro using a previously described chemotaxis assay.22 In brief, migration of cells from the upper to the lower ChemoTX chamber in response to different concentrations of human C5a was determined by adding CyQUANT solution to each lower chamber and measuring the intensity of fluorescence (Migration Signal) of the DNA-binding fluorescent CyQUANT after 120 minutes, which is a relative measure of cell numbers. In vitro, human C5aR responds equally well to murine C5a and human C5a (data not shown), which is in accord with previously reported results.23 The cross-reactivity of CCX168 has been tested against a panel of over 20 chemotactic receptor (including CCR1–10, CXCR1–7, C5L2, C3aR, and ChemR23) and has at least four orders of magnitude less reactivity versus C5aR (data not shown). According to use of a previously described method,24 the effect on in vivo chemotaxis of oral pretreatment 2 hours before intraperitoneal thioglycollate injection with vehicle or a single dose of 30 mg/kg of CCX168 on cell count in peritoneal lavage was measured 24 hours after intraperitoneal injection of thioglycollate. CCX168 effects on C5a-induced leukopenia was studied in hC5aR knock-in mice 1 hour after oral administration of CCX168 by comparing leukocyte counts in blood drawn 1 minute before and 1 minute after intravenous administration of C5a (20 μg/kg). |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In AAV patients receiving 30 mg avacopan twice daily, avacopan had a Cmax of 349 ± 169 ng/mL and an AUC0-12hr of 3466 ± 1921 ng\*h/mL. On this dosing scheme, steady-state plasma concentrations are reached by 13 weeks with a roughly 4-fold accumulation. Co-administration of 30 mg with a high-fat meal increased the Cmax by ~8%, the AUC by ~72%, and delayed the Tmax by four hours (from two hours). Avacopan is mainly eliminated in feces, with smaller amounts present in the urine. Following oral administration of the radiolabeled drug, roughly 77% (7% as unchanged avacopan) was recovered in feces while 10% (<0.1% unchanged) was recovered in urine. Avacopan has an apparent volume of distribution of 345 L. Avacopan has an estimated total apparent body clearance (CL/F) of 16.3 L/h. Metabolism / Metabolites Avacopan is metabolized primarily by CYP3A4. The major circulating M1 metabolite, a mono-hydroxylated form of avacopan, represents ~12% of drug plasma levels and acts as a C5aR antagonist with similar efficacy to avacopan itself. Biological Half-Life A single 30 mg dose of avacopan given with food to healthy subjects resulted in mean elimination half-lives of 97.6 and 55.6 hours for avacopan and its M1 metabolite, respectively. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the clinical use of avacopan during breastfeeding. Because avacopan is over 99% bound to plasma proteins, the amount in milk is likely to be low. If the mother an older infant requires avacopan, it is not a reason to discontinue breastfeeding. but until more data become available, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Avacopan and its M1 metabolite are more than 99.9% bound to plasma proteins. |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Avacopan is a complement 5a receptor (C5aR) antagonist that blocks C5a-induced upregulation of C11b (integrin alpha M) on neutrophils and inhibits C5a-mediated neutrophil activation and migration. Avacopan has been associated with hypersensitivity reactions, including angioedema, and hepatotoxicity, as evidenced by elevated liver transaminases. Likely due to its effect on the complement pathway, avacopan has also been associated with hepatitis B virus reactivation and serious infections, which should be monitored for as appropriate. |
| 分子式 |
C33H35F4N3O2
|
|---|---|
| 分子量 |
581.66
|
| 精确质量 |
581.267
|
| 元素分析 |
C, 68.14; H, 6.07; F, 13.07; N, 7.22; O, 5.50
|
| CAS号 |
1346623-17-3
|
| 相关CAS号 |
1346623-17-3;
|
| PubChem CID |
49841217
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
8.131
|
| tPSA |
61.44
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
42
|
| 分子复杂度/Complexity |
918
|
| 定义原子立体中心数目 |
2
|
| SMILES |
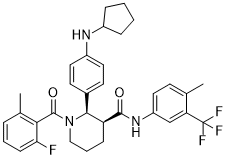
CC1=C(C(=CC=C1)F)C(=O)N2CCC[C@@H]([C@@H]2C3=CC=C(C=C3)NC4CCCC4)C(=O)NC5=CC(=C(C=C5)C)C(F)(F)F
|
| InChi Key |
PUKBOVABABRILL-YZNIXAGQSA-N
|
| InChi Code |
InChI=1S/C33H35F4N3O2/c1-20-12-15-25(19-27(20)33(35,36)37)39-31(41)26-10-6-18-40(32(42)29-21(2)7-5-11-28(29)34)30(26)22-13-16-24(17-14-22)38-23-8-3-4-9-23/h5,7,11-17,19,23,26,30,38H,3-4,6,8-10,18H2,1-2H3,(H,39,41)/t26-,30-/m0/s1
|
| 化学名 |
(2R,3S)-2-[4-(cyclopentylamino)phenyl]-1-(2-fluoro-6-methylbenzoyl)-N-[4-methyl-3-(trifluoromethyl)phenyl]piperidine-3-carboxamide
|
| 别名 |
CCX-168; Tavneos; CCX168; CCX 168. Avacopan
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7192 mL | 8.5961 mL | 17.1922 mL | |
| 5 mM | 0.3438 mL | 1.7192 mL | 3.4384 mL | |
| 10 mM | 0.1719 mL | 0.8596 mL | 1.7192 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。