| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
PKC (IC50 = 0.7 μM)
|
|---|---|
| 体外研究 (In Vitro) |
白屈菜红碱 (IC50: 0.53 uM) 抑制 L-1210 细胞生长 48 小时[1]。白屈菜红碱(0–20 μM,24 小时)可以增加细胞的自噬和自我修复,同时抑制 A549 和 NCI-H1299 细胞的活力。白屈菜红碱(0–5 μM,24 或 48 小时)可导致 SH-SY5Y 细胞过度表达 BclXL。死亡[3]。在 SH-SY5Y 细胞中,白屈菜红碱 (2.5–10 μM, 16) 可导致细胞坏死 [4]。 Chelidonine(0-100 ng/mL,24 小时)可减少 LPS 刺激的初级巨噬细胞中 NO 和 TNF-α 的产生。 Cheerythrine (MIC: 0.156 mg/mL) 对 MRSA、超广谱 β-内酰胺酶金黄色葡萄球菌 (ESBLs-SA) 和革兰氏阳性菌具有抗菌活性。
|
| 体内研究 (In Vivo) |
赤霉素(5 mg/kg,腹腔注射,每日)可以恢复肾功能,并减轻新生血管形成时部分尿路输尿管梗阻(UUO)引起的肾损伤[2]。注射白屈菜赤碱(1-10 mg/kg,腹腔注射)和 100 μg/kg LPS(前 24 小时)后,在 LPS 诱导的中毒性休克中观察到夜间活动率增加、亚硝酸盐和 TNF-α 水平降低以及抗炎作用。小时和1小时)[5]。
|
| 酶活实验 |
二苯并菲啶生物碱白屈菜红碱是大鼠脑中Ca++/磷酸依赖性蛋白激酶(蛋白激酶C:PKC)的强效选择性拮抗剂。激酶的一半最大抑制发生在0.66微M。白屈菜红碱与PKC的催化结构域相互作用,是磷酸受体(组蛋白IIIS)的竞争性抑制剂(Ki=0.7 microM),是ATP的非竞争性抑制剂。白屈菜红碱同样抑制天然PKC及其催化片段,并且不影响[3H]-佛波醇12,13二丁酸酯与PKC的结合,这一事实进一步证明了这种作用。与酪氨酸蛋白激酶、cAMP依赖蛋白激酶和钙/钙调素依赖蛋白激酶相比,白屈菜红碱选择性抑制PKC。在体外测量的芹菜素的强效抗肿瘤活性可能至少部分是由于PKC的抑制,因此表明PKC可能是合理设计抗肿瘤药物的模型。[1]
抗凋亡Bcl-2家族成员的小分子抑制剂的鉴定开辟了新的治疗机会,而天然产物的化学结构和生物活性的巨大多样性尚未得到系统开发。在这里,我们报告了通过高通量筛选107423种天然产物提取物,将白屈菜红碱鉴定为BclXL-Bak Bcl-2同源3(BH3)结构域结合的抑制剂。白屈菜红碱抑制BclXL-Bak BH3肽结合,IC50为1.5微米,并从BclXL中置换含BH3的蛋白Bax。用白屈菜红碱处理的哺乳动物细胞发生凋亡,其特征表明线粒体途径参与其中。虽然星孢菌素、H7、依托泊苷和白屈菜红碱从完整细胞的线粒体释放细胞色素c,但只有白屈菜绿碱从分离的线粒体释放了细胞色素c。此外,本研究中使用的完全抵抗凋亡刺激的BclXL过表达细胞对白屈菜白碱仍然敏感。尽管白屈菜红碱被广泛认为是一种蛋白激酶C抑制剂,但它介导细胞凋亡的机制仍然存在争议。我们的数据表明,白屈菜红碱通过直接靶向Bcl-2家族蛋白的机制触发细胞凋亡[3]。 |
| 细胞实验 |
蛋白质印迹分析 [4]
细胞类型: A549 和 NCI-H1299 细胞 测试浓度: 10、15、20 μM 孵育时间:24小时 实验结果:LC3-II的表达以beclin 1依赖性方式诱导。 |
| 动物实验 |
Animal/Disease Models: Unilateral ureteral obstruction (UUO)-induced neonatal rats [2]
Doses: 5 mg/kg Route of Administration: intraperitoneal (ip) injection, daily Experimental Results: diminished renal damage (increased kidney weight and restored renal function). Inhibits UUO-induced upregulation of renal injury molecule 1 expression, cell apoptosis and renal fibrosis. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Chelerythrine is a potent, selective, and cell-permeable protein kinase C (PKC) inhibitor. It is also the major active natural product found in the plant Zanthoxylum clava-herculis, exhibiting anti-bacterial activity against Staphylococcus aureus. (Wikipedia) Chelerythrine is a selective inhibitor of group A and B PKC isoforms with an antitumor activity. Inhibition of PKC with chelerythrine chloride induces apoptosis by activation of a neutral sphingomyelinase, accumulation of ceramide, and depletion of sphingomyelin. Chelerythrine is at least 100-fold more selective for PKCs than for other kinases. Chelerythrine competes for the conserved catalytic sites of PKC and seems to be a potent and specific inhibitor of the group A and group B kinases. Chelerythrine exhibited cytotoxic activity against nine human tumor cell lines tested in vitro. Radioresistant and chemoresistant squamous cell carcinoma lines (HNSCC) undergo apoptosis rapidly after treatment with chelerythrine in vitro. Chelerythrine treatment of nude mice bearing SQ-20B HNSCC cells is associated with significant tumor growth delay. Also, treatment with chelerythrine resulted in minimal toxicity. (A15441) Toxicity Summary Chelerythrine is a potent, selective, and cell-permeable protein kinase C (PKC) inhibitor. It is also the major active natural product found in the plant Zanthoxylum clava-herculis, exhibiting anti-bacterial activity against Staphylococcus aureus. (Wikipedia) Chelerythrine is a selective inhibitor of group A and B PKC isoforms with an antitumor activity. Inhibition of PKC with chelerythrine chloride induces apoptosis by activation of a neutral sphingomyelinase, accumulation of ceramide, and depletion of sphingomyelin. Chelerythrine is at least 100-fold more selective for PKCs than for other kinases. Chelerythrine competes for the conserved catalytic sites of PKC and seems to be a potent and specific inhibitor of the group A and group B kinases. Chelerythrine exhibited cytotoxic activity against nine human tumor cell lines tested in vitro. Radioresistant and chemoresistant squamous cell carcinoma lines (HNSCC) undergo apoptosis rapidly after treatment with chelerythrine in vitro. Chelerythrine treatment of nude mice bearing SQ-20B HNSCC cells is associated with significant tumor growth delay. Also, treatment with chelerythrine resulted in minimal toxicity. |
| 参考文献 |
|
| 其他信息 |
Chelerythrine is a benzophenanthridine alkaloid isolated from the root of Zanthoxylum simulans, Chelidonium majus L., and other Papaveraceae. It has a role as an EC 2.7.11.13 (protein kinase C) inhibitor, an antibacterial agent and an antineoplastic agent. It is a benzophenanthridine alkaloid and an organic cation.
A benzophenanthridine alkaloid evaluated as a kinase-inhibitor. Chelerythrine has been reported in Corydalis ternata, Zanthoxylum simulans, and other organisms with data available. Chelerythrine is a benzophenanthridine alkaloid extracted from the plant Greater celandine (Chelidonium majus). It is a potent, selective, and cell-permeable protein kinase C inhibitor. See also: Sanguinaria canadensis root (part of); Chelidonium majus flowering top (part of). The present study aimed to evaluate the renoprotective effects of chelerythrine (CHE), a protein kinase C inhibitor, on neonatal rats after partial unilateral ureteral obstruction (UUO) surgery. New born Sprague Dawley rats were subjected to partial UUO 48 h after birth and received a daily intraperitoneal injection of 5 mg/kg CHE. At 21-day age, the rats were scarified and the kidneys were collected for analysis. Results showed that CHE treatment significantly increased kidney weight and restored renal function in the obstructed kidney. Histological examination demonstrated that CHE attenuated renal injury by reducing renal parenchymal loss and preventing glomerular and tubular degeneration. In addition, CHE inhibited partial UUO-induced upregulated kidney injury molecule-1 expression and apoptosis and renal fibrosis. Moreover, as a PKC inhibitor, CHE significantly inhibited PKCα and PKCβ membrane translocation. This action may be associated with its effects of anti-apoptosis and anti-fibrosis and contribute to the renoprotection. This short-term study suggests that CHE is beneficial for obstructive nephropathy in neonatal rats and provides foundation for further studies to reveal the long-term effects of CHE on obstructive nephropathy in children and infants.[2] Chelerythrine (CHE), a natural benzo[c]phenanthridine alkaloid, shows anti-cancer effect through a number of mechanisms. Herein, the effect and mechanism of the CHE-induced autophagy, a type II programmed cell death, in non-small cell lung cancer (NSCLC) cells were studied for the first time. CHE induced cell viability decrease, colony formation inhibition, and apoptosis in a concentration-dependent manner in NSCLC A549 and NCI-H1299 cells. In addition, CHE triggered the expression of phosphatidylethanolamine-modified microtubule-associated protein light-chain 3 (LC3-II). The CHE-induced expression of LC3-II was further increased in the combination treatment with chloroquine (CQ), an autophagy inhibitor, and large amounts of red-puncta were observed in the CHE-treated A549 cells with stable expression of mRFP-EGFP-LC3, indicating that CHE induces autophagy flux. Silence of beclin 1 reversed the CHE-induced expression of LC3-II. Inhibition of autophagy remarkably reversed the CHE-induced cell viability decrease and apoptosis in NCI-H1299 cells but not in A549 cells. Furthermore, CHE triggered reactive oxygen species (ROS) generation in both cell lines. A decreased level of ROS through pretreatment with N-acetyl-L-cysteine reversed the CHE-induced cell viability decrease, apoptosis, and autophagy. Taken together, CHE induced distinctive autophagy in A549 (accompanied autophagy) and NCI-H1299 (pro-death autophagy) cells and a decreased level of ROS reversed the effect of CHE in NSCLC cells in terms of cell viability, apoptosis, and autophagy.[4] A quaternary benzo [c] alkaloid chelerythrine (CHE), which is a traditional herbal prescription, has been used for the treatment of various inflammatory diseases. To gain insight into the anti-inflammatory effect and molecular mechanisms underlying the anti-inflammatory activity of CHE, we used experimentally induced mice endotoxic shock moled and lipopolysaccharide (LPS)-induced murine peritoneal macrophages to examine the anti-inflammatory function of CHE. CHE displayed significant anti-inflammatory effects in experimentally induced mice endotoxic shock model in vivo through inhibition of LPS-induced tumor necrosis factor-alpha (TNF-α) level and nitric oxide (NO) production in serum. Additionally, our data suggest that CHE treatment inhibits LPS-induced TNF-α level and NO production in LPS-induced murine peritoneal macrophages through selective inhibition of p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) activation. Moreover, the effects of CHE on NO and cytokine TNF-α production can possibly be explained by the role of p38 MAPK and ERK1/2 in the regulation of inflammatory mediators expression.[5] |
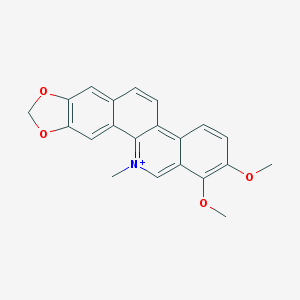
| 分子式 |
C21H18NO4
|
|---|---|
| 分子量 |
348.3719
|
| 精确质量 |
348.123
|
| 元素分析 |
C, 72.40; H, 5.21; N, 4.02; O, 18.37
|
| CAS号 |
34316-15-9
|
| 相关CAS号 |
Chelerythrine chloride;3895-92-9; 478-03-5 (OH-); 3895-92-9 (chloride); 34316-15-9
|
| PubChem CID |
2703
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 熔点 |
195-205ºC
|
| LogP |
0.72
|
| tPSA |
40.8
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
516
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O1C([H])([H])OC2=C1C([H])=C1C(=C2[H])C([H])=C([H])C2=C3C([H])=C([H])C(=C(C3=C([H])[N+](C([H])([H])[H])=C21)OC([H])([H])[H])OC([H])([H])[H]
|
| InChi Key |
LLEJIEBFSOEYIV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H18NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-10H,11H2,1-3H3/q+1
|
| 化学名 |
1,2-dimethoxy-12-methyl-[1,3]benzodioxolo[5,6-c]phenanthridin-12-ium
|
| 别名 |
Toddalin; cheleritrine; Toddaline; broussonpapyrine; [1,3]Benzodioxolo[5,6-c]phenanthridinium, 1,2-dimethoxy-12-methyl-; EINECS 251-930-0; Chelerythrine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8705 mL | 14.3526 mL | 28.7051 mL | |
| 5 mM | 0.5741 mL | 2.8705 mL | 5.7410 mL | |
| 10 mM | 0.2871 mL | 1.4353 mL | 2.8705 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。